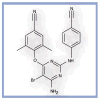
Etravirine: a second-generation NNRTI for treatment-experienced adults with resistant HIV-1 infection
- PMID: 19881888
- PMCID: PMC2770189
- DOI: 10.2217/17469600.2.6.525
Etravirine: a second-generation NNRTI for treatment-experienced adults with resistant HIV-1 infection
Abstract
Etravirine, a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), was approved in the USA in January, 2008, with approval in Europe expected later this year. It is dosed at 200 mg (two 100 mg tablets) twice daily foll owing a meal. It is approved for treatment of HIV-1 infection in adults failing a stable antiretroviral regimen with resistance to other NNRTIs and other antiretroviral agents. Etravirine is active against HIV with single mutations in the reverse transcriptase (e.g., K103N) that confer class resistance to first-generation NNRTIs. Clinical efficacy in Phase III trials has been demonstrated for up to 48 weeks of follow-up. In these Phase III trials, rash was the only adverse event that was significantly more prevalent with etravirine than with placebo. Etravirine has a tolerability and safety profile comparable to placebo with the exception of rash. Rash was generally grade 1 or 2, was not associated with prior NNRTI-related rash, was more common in women than in men, appeared a median of 12 days after treatment initiation and resolved spontaneously with continued therapy. Etravirine is the first agent in the NNRTI class that can be used for HIV-1 virus with resistance to other NNRTIs owing to a higher genetic barrier to resistance.
References
Bibliography
-
- Tassie JM, Grabar S, Lancar R, Deloumeaux J, Bentata M, Costagliola D. Time to AIDS from 1992 to 1999 in HIV-1 infected subjects with known dates of infection. J Acquir Immune Defic Syndr. 2002;30(1):81–87. - PubMed
-
• Description of the changing face of HIV/AIDS care in the era of combination antiretroviral therapy with the goal of virologic suppression.
-
- Schneider MF, Gange SJ, Williams CM, et al. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS. 2005;19:2009–2018. - PubMed
-
- Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 Recommendations of the International AIDS Society–USA Panel. JAMA. 2008;300:555–570. - PubMed
-
- Wainberg MA. HIV resistance to nevirapine and other non-nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2003;34:S2–S7. - PubMed
-
- Deeks SJ. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr. 2001;26:S25–S33. - PubMed
-
• Highlights the mechanism of HIV-1 resistance to the first-generation nonnucleoside reverse transcriptase inhibitors (NNRTIs) and the benefits and limitations of treatment regimens incorporating the older NNRTIs.
Websites
-
- British HIV Association recommendations. [November 17 2008]. www.bhiva.org/files/file1030835.pdf.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. U.S. Department of Health and Human Services; Jan 172008. [November 17 2008]. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents; pp. 1–128. http://aidsinfo.nih.gov/contentfiles/AA_Tables.pdf.
-
- US Food and Drug Administration. [November 17 2008]. http://www.fda.gov/cder/rdmt/InternetNDA08.htm.
Grants and funding
LinkOut - more resources
Full Text Sources