Induction of type I interferon by RNA viruses: cellular receptors and their substrates
- PMID: 19882216
- PMCID: PMC2860555
- DOI: 10.1007/s00726-009-0374-0
Induction of type I interferon by RNA viruses: cellular receptors and their substrates
Erratum in
- Amino Acids. 2011 Apr;40(4):1263
Abstract
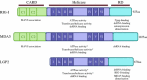
Virus recognition and induction of interferon (IFN) are critical components of the innate immune system. The Toll-like receptor (TLR) and RIG-I-like receptor families have been characterized as key players in RNA virus detection. Signaling cascades initiated by these receptors are crucial for establishment of an IFN signaling mediated antiviral state in infected and neighboring cells and containment of virus replication as well as initiation of the adaptive immune response. In this review, we focus on the diverse and overlapping functions of these receptors, their physiological importance, and respective viral inducers. We highlight the roles of TRL3, TLR7/8, retinoic acid inducible gene I, melanoma differentiation-associated gene 5, and the RNA molecules responsible for activating these viral sensors.
Figures

References
-
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19AI62623/AI/NIAID NIH HHS/United States
- U01 AI070469/AI/NIAID NIH HHS/United States
- R01 AI046954/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- U19 AI062623/AI/NIAID NIH HHS/United States
- U54 AI057168/AI/NIAID NIH HHS/United States
- U01AI70469/AI/NIAID NIH HHS/United States
- U19 AI083025/AI/NIAID NIH HHS/United States
- U54AI57168/AI/NIAID NIH HHS/United States
- P01AI58113/AI/NIAID NIH HHS/United States
- P01AI82325/AI/NIAID NIH HHS/United States
- U19AI83025/AI/NIAID NIH HHS/United States
- P01 AI082325/AI/NIAID NIH HHS/United States
- R01AI46954/AI/NIAID NIH HHS/United States
- HHSN266200700010C/AI/NIAID NIH HHS/United States
- P01 AI058113/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

