Intravenous immunoglobulins--understanding properties and mechanisms
- PMID: 19883419
- PMCID: PMC2801035
- DOI: 10.1111/j.1365-2249.2009.04022.x
Intravenous immunoglobulins--understanding properties and mechanisms
Abstract
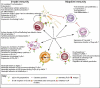
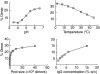
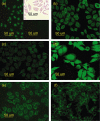
High-dose intravenous immunoglobulin (IVIg) preparations are used currently for the treatment of autoimmune or inflammatory diseases. Despite numerous studies demonstrating efficacy, the precise mode of action of IVIg remains unclear. Paradoxically, IgG can exert both pro- and anti-inflammatory activities, depending on its concentration. The proinflammatory activity of low-dose IVIg requires complement activation or binding of the Fc fragment of IgG to IgG-specific receptors (FcgammaR) on innate immune effector cells. In contrast, when administered in high concentrations, IVIg has anti-inflammatory properties. How this anti-inflammatory effect is mediated has not yet been elucidated fully, and several mutually non-exclusive mechanisms have been proposed. This paper represents the proceedings of a session entitled 'IVIg--Understanding properties and mechanisms' at the 6th International Immunoglobulin Symposium that was held in Interlaken on 26-28 March 2009. The presentations addressed how IgG may affect the cellular compartment, evidence for IVIg-mediated scavenging of complement fragments, the role of the dimeric fraction of IVIg, the anti-inflammatory properties of the minor fraction of sialylated IgG molecules, and the genetic organization and variation in FcgammaRs. These findings demonstrate the considerable progress that has been made in understanding the mechanisms of action of IVIgs, and may influence future perspectives in the field of Ig therapy.
Figures

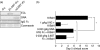

References
-
- Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. - PubMed
-
- Conley ME, Dobbs AK, Farmer DM, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. - PubMed
-
- Durandy A, Wahn V, Petteway S, Gelfand EW. Immunoglobulin replacement therapy in primary antibody deficiency diseases – maximizing success. Int Arch Allergy Immunol. 2005;136:217–29. - PubMed
-
- Gürcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in autoimmune and chronic inflammatory disorders. Ann Pharmacother. 2007;41:812–23. - PubMed
-
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

