Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force
- PMID: 19883577
- PMCID: PMC2773556
- DOI: 10.1016/j.jss.2008.06.029
Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force
Abstract
Background: Adipose tissue is a readily available source of multipotent adult stem cells for use in tissue engineering/regenerative medicine. Various growth factors have been used to stimulate acquisition of endothelial characteristics by adipose-derived stem cells (ASC). Herein we study the effects of endothelial cell growth supplement (ECGS) and physiological shear force on the differentiation of ASC into endothelial cells.
Materials and methods: Human ASC (CD13(+)29(+)90(+)31(-)45(-)) were isolated from periumbilical fat, cultured in ECGS media (for up to 3 wk), and exposed to physiological shear force (12 dynes for up to 8 d) in vitro. Endothelial phenotype was defined by cord formation on Matrigel, acetylated-low density lipoprotein (acLDL) uptake, and expression of nitric oxide synthase (eNOS), von Willebrand factor (vWF), and CD31 (platelet endothelial cell adhesion molecule, PECAM). Additionally, cell thrombogenicity was evaluated by seeding canine autologous ASC onto vascular grafts implanted within the canine arterial circulation for 2 wk.
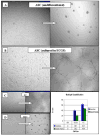
Results: We found that undifferentiated ASC did not display any of the noted endothelial characteristics. After culture in ECGS, ASC formed cords in Matrigel but failed to take up acLDL or express the molecular markers. Subsequent exposure to shear resulted in stem cell realignment, acLDL uptake, and expression of CD31; eNOS and vWF expression was still not observed. Grafts seeded with cells grown in ECGS (+/- shear) remained patent (six of seven) at 2 wk but had a thin coat of fibrin along the luminal surfaces.
Conclusions: This study suggests that (1) ECGS and shear promote the expression of several endothelial characteristics in human adipose-derived stem cells, but not eNOS or vWF; (2) their combined effects appear synergistic; and (3) stem cells differentiated in ECGS appear mildly thrombogenic in vitro, possibly related, in part, to insufficient eNOS expression. Thus, while the acquisition of several endothelial characteristics by adult stem cells derived from adipose tissue suggests these cells are a viable source of autologous cells for cardiovascular regeneration, further stimulation/modifications are necessary prior to using them as a true endothelial cell replacement.
Figures



References
-
- Fukuda K, Yuasa S. Stem Cells as a Source of Regenerative Cardiomyocytes. Circ Res. 2006;98:1002–1013. - PubMed
-
- Wollert KC, Drexler H. Clinical Applications of Stem Cells for the Heart. Circ Res. 2005;96:151–163. - PubMed
-
- Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. Journal of Molecular and Cellular Cardiology. 2005;39:733–742. - PubMed
-
- Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovascular Research. 2005;66:543–551. - PubMed
-
- Campbell GR, Campbell JH. Development of tissue engineered vascular grafts. Curr Pharm Biotechnol. 2007;8:43–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

