Modeling species-specific diacylglycerol dynamics in the RAW 264.7 macrophage
- PMID: 19883664
- PMCID: PMC2822135
- DOI: 10.1016/j.jtbi.2009.10.030
Modeling species-specific diacylglycerol dynamics in the RAW 264.7 macrophage
Abstract
A mathematical model of the G protein signaling pathway in RAW 264.7 macrophages downstream of P2Y(6) receptors activated by the ubiquitous signaling nucleotide uridine 5'-diphosphate is developed. The model, which is based on time-course measurements of inositol trisphosphate, cytosolic calcium, and diacylglycerol, focuses particularly on differential dynamics of multiple chemical species of diacylglycerol. When using the canonical pathway representation, the model predicted that key interactions were missing from the current network structure. Indeed, the model suggested that accurate depiction of experimental observations required an additional branch to the signaling pathway. An intracellular pool of diacylglycerol is immediately phosphorylated upon stimulation of an extracellular receptor for uridine 5'-diphosphate and subsequently used to aid replenishment of phosphatidylinositol. As a result of sensitivity analysis of the model parameters, key predictions can be made regarding which of these parameters are the most sensitive to perturbations and are therefore most responsible for output uncertainty.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures







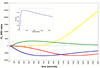
 ), receptor recycling rate, kr (
), receptor recycling rate, kr ( ), PIP2 replenishment rate, krep (
), PIP2 replenishment rate, krep ( ), and IP3 degradation rate, kd3 (
), and IP3 degradation rate, kd3 ( ). Inset: values for all time points are ≥ 0.8, enusuring that the SRCs for IP3 are good measures of sensitivity.
). Inset: values for all time points are ≥ 0.8, enusuring that the SRCs for IP3 are good measures of sensitivity.
 ), production rate of DAGp2, kap2 (
), production rate of DAGp2, kap2 ( ), degradation rate of DAGp2, kdp2 (
), degradation rate of DAGp2, kdp2 ( ), receptor rate of phosphorylation, kp (
), receptor rate of phosphorylation, kp ( ), and receptor recycling rate, kr (
), and receptor recycling rate, kr ( ). Inset: Since values for all time points are ≥ 0.95, the SRCs for DAG are good measures of sensitivity.
). Inset: Since values for all time points are ≥ 0.95, the SRCs for DAG are good measures of sensitivity.


References
-
- Alliance for Cellular Signaling website. 2008 URL http://www.signaling-gateway.org/.
-
- Bishop WR, Bell RM. Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation. Oncogene Res. 1988;2(3):205–218. - PubMed
-
- Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors: Ser-333 and Ser-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J. Biol. Chem. 2001;276(15):11939–11948. - PubMed
-
- Callender HL. Ph. D. thesis. Vanderbilt University; 2007. Mathematical modeling of species-specific diacylglycerol dynamics in the RAW 264.7 macrophage following P2Y6 receptor activation by uridine 5’-diphosphate. URL http://etd.library.vanderbilt.edu/ETD-db/available/etd-06212007-223551/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

