GSK3: a multifaceted kinase in Wnt signaling
- PMID: 19884009
- PMCID: PMC2834833
- DOI: 10.1016/j.tibs.2009.10.002
GSK3: a multifaceted kinase in Wnt signaling
Abstract
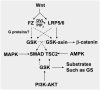
GSK3 is one of the few signaling mediators that play central roles in a diverse range of signaling pathways, including those activated by Wnts, hedgehog, growth factors, cytokines, and G protein-coupled ligands. Although the inhibition of GSK3-mediated beta-catenin phosphorylation is known to be the key event in Wnt-beta-catenin signaling, the mechanisms that underlie this event remain incompletely understood. The recent demonstration of GSK3 involvement in Wnt receptor phosphorylation illustrates the multifaceted roles that GSK3 plays in Wnt-beta-catenin signaling. In this review, we will summarize these recent results and offer explanations, hypotheses, and models to reconcile some of these observations.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures


References
-
- Embi N, et al. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. - PubMed
-
- Wada A. GSK-3 inhibitors and insulin receptor signaling in health, disease, and therapeutics. Front Biosci. 2009;14:1558–1570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

