Application of proteomic marker ensembles to subcellular organelle identification
- PMID: 19884172
- PMCID: PMC2830848
- DOI: 10.1074/mcp.M900432-MCP200
Application of proteomic marker ensembles to subcellular organelle identification
Abstract
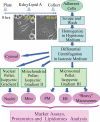
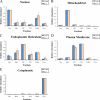
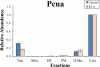
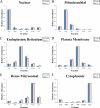
Compartmentalization of biological processes and the associated cellular components is crucial for cell function. Typically, the location of a component is revealed through a co-localization and/or co-purification with an organelle marker. Therefore, the identification of reliable markers is critical for a thorough understanding of cellular function and dysfunction. We fractionated macrophage-like RAW264.7 cells, both in the resting and endotoxin-activated states, into six fractions representing the major organelles/compartments: nuclei, mitochondria, cytoplasm, endoplasmic reticulum, and plasma membrane as well as an additional dense microsomal fraction. The identity of the first five of these fractions was confirmed via the distribution of conventional enzymatic markers. Through a quantitative liquid chromatography/mass spectrometry-based proteomics analysis of the fractions, we identified 50-member ensembles of marker proteins ("marker ensembles") specific for each of the corresponding organelles/compartments. Our analysis attributed 206 of the 250 marker proteins ( approximately 82%) to organelles that are consistent with the location annotations in the public domain (obtained using DAVID 2008, EntrezGene, Swiss-Prot, and references therein). Moreover, we were able to correct locations for a subset of the remaining proteins, thus proving the superior power of analysis using multiple organelles as compared with an analysis using one specific organelle. The marker ensembles were used to calculate the organelle composition of the six above mentioned subcellular fractions. Knowledge of the precise composition of these fractions can be used to calculate the levels of metabolites in the pure organelles. As a proof of principle, we applied these calculations to known mitochondria-specific lipids (cardiolipins and ubiquinones) and demonstrated their exclusive mitochondrial location. We speculate that the organelle-specific protein ensembles may be used to systematically redefine originally morphologically defined organelles as biochemical entities.
Figures




References
-
- Yates J. R., 3rd, Gilchrist A., Howell K. E., Bergeron J. J. ( 2005) Proteomics of organelles and large cellular structures. Nat. Rev. Mol. Cell Biol 6, 702– 714 - PubMed
-
- Forner F., Foster L. J., Campanaro S., Valle G., Mann M. ( 2006) Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol. Cell. Proteomics 5, 608– 619 - PubMed
-
- Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. ( 2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426, 570– 574 - PubMed
-
- Dunkley T. P., Watson R., Griffin J. L., Dupree P., Lilley K. S. ( 2004) Localization of organelle proteins by isotope tagging (LOPIT). Mol. Cell. Proteomics 3, 1128– 1134 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

