Both synaptic and intrinsic mechanisms underlie the different properties of population bursts in the hippocampal CA3 area of immature versus adult rats
- PMID: 19884320
- PMCID: PMC2808548
- DOI: 10.1113/jphysiol.2009.179887
Both synaptic and intrinsic mechanisms underlie the different properties of population bursts in the hippocampal CA3 area of immature versus adult rats
Abstract
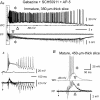
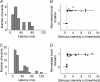
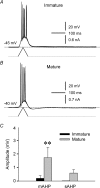
Pharmacological blockade of GABA(A) receptors on CA3 pyramidal cells in hippocampal slices from immature rats (i.e. second to third postnatal weeks), compared to CA3 slices from adult rats, is known to cause prolonged burst discharges (i.e. several seconds vs. tens of milliseconds). Synaptic and intrinsic mechanisms responsible for this developmental difference in burst duration were analysed in isolated minislices of the CA3 area. The frequency and amplitude of spontaneous EPSCs in CA3 pyramidal cells were greater in slices from immature than mature rats. In the presence of GABA(A)- and GABA(B)-receptor antagonists, the burst discharges of immature CA3 pyramidal cells were still prolonged in thinner slices (350 microm, vs. 450 microm in adults, to compensate for developmental differences in neuronal density) and in NMDA- and mGlu1-receptor antagonists. The AMPA receptor antagonist DNQX blocked the remaining burst discharges, suggesting that differences in recurrent excitatory circuits contributed to the prolonged bursts of immature CA3 pyramidal cells. In slices from immature versus adult rats, the CA3 recurrent synaptic responses showed potentiation to repetitive stimulation, suggestive of a lower transmitter release probability. The intrinsic firing ability was greater in CA3 pyramidal neurons from immature than adult rats, and the medium-duration afterhyperpolarization was smaller. These data suggest that, compared to adults, the CA3 area of immature rats contains a more robust recurrent excitatory synaptic network, greater intrinsic membrane excitability, and an increased capacity for sustained transmitter release, which together may account for the more prolonged network bursts in immature versus adult CA3.
Figures






Comment in
-
Immature brains don't need GABA to get 'hyper'-excited.J Physiol. 2010 Jan 1;588(Pt 1):7-8. doi: 10.1113/jphysiol.2009.183905. J Physiol. 2010. PMID: 20045897 Free PMC article. No abstract available.
References
-
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84:2204–2216. - PubMed
-
- Ben-Ari Y, Cherubini E, Krnjevic K. Changes in voltage dependence of NMDA currents during development. Neurosci Lett. 1988;94:88–92. - PubMed
-
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. - PubMed
-
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

