HDAC6 is a target for protection and regeneration following injury in the nervous system
- PMID: 19884510
- PMCID: PMC2780768
- DOI: 10.1073/pnas.0907935106
HDAC6 is a target for protection and regeneration following injury in the nervous system
Abstract
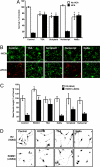
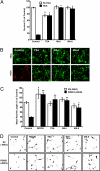
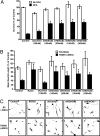
Central nervous system (CNS) trauma can result in tissue disruption, neuronal and axonal degeneration, and neurological dysfunction. The limited spontaneous CNS repair in adulthood and aging is often insufficient to overcome disability. Several investigations have demonstrated that targeting HDAC activity can protect neurons and glia and improve outcomes in CNS injury and disease models. However, the enthusiasm for pan-HDAC inhibition in treating neurological conditions is tempered by their toxicity toward a host of CNS cell types -a biological extension of their anticancer properties. Identification of the HDAC isoform, or isoforms, that specifically mediate the beneficial effects of pan-HDAC inhibition could overcome this concern. Here, we show that pan-HDAC inhibition not only promotes neuronal protection against oxidative stress, a common mediator of injury in many neurological conditions, but also promotes neurite growth on myelin-associated glycoprotein and chondroitin sulfate proteoglycan substrates. Real-time PCR revealed a robust and selective increase in HDAC6 expression due to injury in neurons. Accordingly, we have used pharmacological and genetic approaches to demonstrate that inhibition of HDAC6 can promote survival and regeneration of neurons. Consistent with a cytoplasmic localization, the biological effects of HDAC6 inhibition appear transcription-independent. Notably, we find that selective inhibition of HDAC6 avoids cell death associated with pan-HDAC inhibition. Together, these findings define HDAC6 as a potential nontoxic therapeutic target for ameliorating CNS injury characterized by oxidative stress-induced neurodegeneration and insufficient axonal regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Anderson MF, Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem. 2002;81:541–549. - PubMed
-
- Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11(Suppl 1):13–22. - PubMed
-
- Springer JE. Apoptotic cell death following traumatic injury to the central nervous system. J Biochem Mol Biol. 2002;35:94–105. - PubMed
-
- Blight AR. Cellular morphology of chronic spinal cord injury in the cat: Analysis of myelinated axons by line-sampling. Neuroscience. 1983;10:521–543. - PubMed
-
- Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

