Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages
- PMID: 19884654
- PMCID: PMC2786804
- DOI: 10.1172/JCI40053
Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages
Abstract
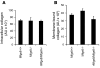
Milk fat globule epidermal growth factor 8 (Mfge8) is a soluble glycoprotein known to regulate inflammation and immunity by mediating apoptotic cell clearance. Since fibrosis can occur as a result of exaggerated apoptosis and inflammation, we set out to investigate the hypothesis that Mfge8 might negatively regulate tissue fibrosis. We report here that Mfge8 does decrease the severity of tissue fibrosis in a mouse model of pulmonary fibrosis; however, it does so not through effects on inflammation and apoptotic cell clearance, but by binding and targeting collagen for cellular uptake through its discoidin domains. Initial analysis revealed that Mfge8-/- mice exhibited enhanced pulmonary fibrosis after bleomycin-induced lung injury. However, they did not have increased inflammation or impaired apoptotic cell clearance after lung injury compared with Mfge8+/+ mice; rather, they had a defect in collagen turnover. Further experiments indicated that Mfge8 directly bound collagen and that Mfge8-/- macrophages exhibited defective collagen uptake that could be rescued by recombinant Mfge8 containing at least one discoidin domain. These data demonstrate a critical role for Mfge8 in decreasing the severity of murine tissue fibrosis by facilitating the removal of accumulated collagen.
Figures

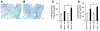

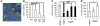


References
-
- Raghu G., Striker L.J., Hudson L.D., Striker G.E. Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Respir. Dis. 1985;131:281–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL053949/HL/NHLBI NIH HHS/United States
- HL53949/HL/NHLBI NIH HHS/United States
- S10 RR023443/RR/NCRR NIH HHS/United States
- R01 HL053949/HL/NHLBI NIH HHS/United States
- HL083950/HL/NHLBI NIH HHS/United States
- AI024674/AI/NIAID NIH HHS/United States
- R01 AI024674/AI/NIAID NIH HHS/United States
- HL083985/HL/NHLBI NIH HHS/United States
- R37 AI024674/AI/NIAID NIH HHS/United States
- P01 AI053194/AI/NIAID NIH HHS/United States
- HL66600/HL/NHLBI NIH HHS/United States
- K08 HL083985/HL/NHLBI NIH HHS/United States
- U01 HL066600/HL/NHLBI NIH HHS/United States
- HL64353/HL/NHLBI NIH HHS/United States
- R01 HL083950/HL/NHLBI NIH HHS/United States
- AI053194/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

