Acromegaly pathogenesis and treatment
- PMID: 19884662
- PMCID: PMC2769196
- DOI: 10.1172/JCI39375
Acromegaly pathogenesis and treatment
Abstract
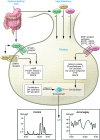
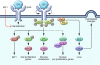
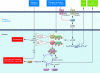
Dysregulated growth hormone (GH) hypersecretion is usually caused by a GH-secreting pituitary adenoma and leads to acromegaly - a disorder of disproportionate skeletal, tissue, and organ growth. High GH and IGF1 levels lead to comorbidities including arthritis, facial changes, prognathism, and glucose intolerance. If the condition is untreated, enhanced mortality due to cardiovascular, cerebrovascular, and pulmonary dysfunction is associated with a 30% decrease in life span. This Review discusses acromegaly pathogenesis and management options. The latter include surgery, radiation, and use of novel medications. Somatostatin receptor (SSTR) ligands inhibit GH release, control tumor growth, and attenuate peripheral GH action, while GH receptor antagonists block GH action and effectively lower IGF1 levels. Novel peptides, including SSTR ligands, exhibiting polyreceptor subtype affinities and chimeric dopaminergic-somatostatinergic properties are currently in clinical trials. Effective control of GH and IGF1 hypersecretion and ablation or stabilization of the pituitary tumor mass lead to improved comorbidities and lowering of mortality rates for this hormonal disorder.
Figures

References
-
- Molitch M.E. Clinical manifestations of acromegaly. Endocrinol. Metab. Clin. North Am. . 1992;21:597–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

