Endpoints in phase II trials for advanced non-small cell lung cancer
- PMID: 19884856
- PMCID: PMC2798014
- DOI: 10.1097/JTO.0b013e3181c0a313
Endpoints in phase II trials for advanced non-small cell lung cancer
Abstract
Introduction: We investigated the relationships between progression-free survival (PFS), response, confirmed response, and failure-free survival (FFS) with overall survival (OS) to assess their suitability as primary endpoints in phase II trials for advanced non-small cell lung cancer.
Methods: Individual data of 284 patients from four phase II trials were pooled. Progression status and response were modeled as time dependent variables in a multivariable (adjusted for baseline age, gender, stage, and performance status) Cox proportional hazards model for OS, stratified by trial. Subsequently, Cox proportional hazards models were used to assess the impact of PFS, response, confirmed response, and FFS on subsequent survival, using landmark analysis at 8, 12, 16, 20, and 24 weeks. Model discrimination was evaluated using the concordance index (c-index).
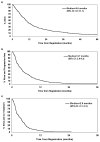
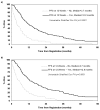
Results: The overall median OS, PFS, and FFS were 9.6, 3.7, and 2.8 months, and the response and confirmed response rates were 21 and 15%, respectively. Both progression status and response as time dependent covariates were significantly associated with OS (p < 0.0001; p = 0.009). PFS and FFS at 12 weeks significantly predicted for subsequent survival with the strongest c-index and hazard ratio combination in landmark analyses (hazard ratio, c-index: PFS: 0.39, 0.67; FFS: 0.37, 0.67). The c-indices for response and confirmed response were low (0.59-0.60), indicating their inability to sufficiently discriminate subsequent patient survival outcomes.
Conclusions: FFS or PFS at 12 weeks is a stronger predictor of subsequent patient survival compared with tumor response and should be routinely used as endpoints in phase II trials for advanced non-small cell lung cancer.
Figures
References
-
- NCI. Cancer Statistics Review. :1975–2002.
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–50. - PubMed
-
- Kim ES, H, Moon J, Crowley J, et al. S0536: SWOG phase II trial of carboplatin (C), paclitaxel (P), cetuximab (CX) and bevacizumab (B) followed by cetuximab and bevacizumab in advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2008;3(11) 4:S266.
-
- Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–82. - PubMed
Publication types
MeSH terms
Grants and funding
- U10 CA052352/CA/NCI NIH HHS/United States
- U10 CA037417/CA/NCI NIH HHS/United States
- CA-52352/CA/NCI NIH HHS/United States
- U10 CA037404/CA/NCI NIH HHS/United States
- U10 CA035448/CA/NCI NIH HHS/United States
- CA-35103/CA/NCI NIH HHS/United States
- CA-25224/CA/NCI NIH HHS/United States
- U10 CA035272/CA/NCI NIH HHS/United States
- CA-35415/CA/NCI NIH HHS/United States
- CA-63849/CA/NCI NIH HHS/United States
- U10 CA035103/CA/NCI NIH HHS/United States
- U10 CA035269/CA/NCI NIH HHS/United States
- CA-37404/CA/NCI NIH HHS/United States
- CA-35113/CA/NCI NIH HHS/United States
- U10 CA035101/CA/NCI NIH HHS/United States
- U10 CA035113/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- U10 CA035415/CA/NCI NIH HHS/United States
- U10 CA063849/CA/NCI NIH HHS/United States
- CA-37417/CA/NCI NIH HHS/United States
- CA-35272/CA/NCI NIH HHS/United States
- CA-35269/CA/NCI NIH HHS/United States
- CA-35448/CA/NCI NIH HHS/United States
- CA-35101/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical