An electronic protocol for translation of research results to clinical practice: a preliminary report
- PMID: 19885263
- PMCID: PMC2769803
- DOI: 10.1177/193229680800200508
An electronic protocol for translation of research results to clinical practice: a preliminary report
Abstract
Introduction: We evaluated the feasibility of using an electronic protocol developed for research use (Research-eProtocol-insulin) for blood glucose management in usual intensive care unit clinical practice.
Methods: We implemented the rules of Research-eProtocol-insulin in the electronic medical record of the Intermountain Healthcare hospital system (Clinical-eProtocol-insulin) for use in usual clinical practice. We evaluated the performance of Clinical-eProtocol-insulin rules in the intensive care units of seven Intermountain Healthcare hospitals and compared this performance with the performance of Research-eProtocol-insulin at the LDS Hospital Shock/Trauma/Respiratory Intensive Care Unit.
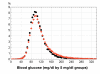
Results: Clinician (nurse or physician) compliance with computerized protocol recommendations was 95% (of 21,325 recommendations) with Research-eProtocol-insulin and 92% (of 109,458 recommendations) with Clinical-eProtocol-insulin. The blood glucose distribution in clinical practice (Clinical-eProtocol-insulin) was similar to the research use distribution (Research-eProtocol-insulin); however, the mean values (119 mg/dl vs 113 mg/dl) were statistically different (P = 0.0001). Hypoglycemia rates in the research and practice settings did not differ: the percentage of measurements < or =40 mg/dl (0.11% vs 0.1%, P = 0.65) and the percentage of patients with at least one blood glucose < or =40 mg/dl (4.2% vs 3%, P = 0.23) were not statistically significantly different.
Conclusion: Our electronic blood glucose protocol enabled translation of a research decision-support tool (Research-eProtocol-insulin) to usual clinical practice (Clinical-eProtocol-insulin).
Keywords: clinical; clinical protocol; computer protocol; glucose; intensive care; replicability.
Figures

References
-
- Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28(1):413–433. - PubMed
-
- Campbell D, Stanley J. Experimental and quasi-experimental designs for research. Boston: Houghton Mifflin Co.; 1966. (reprinted from Handbook of Research on Teaching, 1963)
-
- Babbie E. Survey research methods. Belmont, CA: Wadsworth Publishing Co.; 1990.
-
- Guyatt G, Sackett D, Cook D. User's guide to the medical literature. II. How to use an article about therapy or prevention; A. Are the results of the study valid? JAMA. 1993;270(21):2598–2601. - PubMed
LinkOut - more resources
Full Text Sources

