Identification and functional characterization of N-terminally acetylated proteins in Drosophila melanogaster
- PMID: 19885390
- PMCID: PMC2762599
- DOI: 10.1371/journal.pbio.1000236
Identification and functional characterization of N-terminally acetylated proteins in Drosophila melanogaster
Abstract
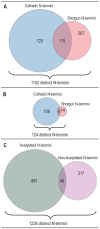
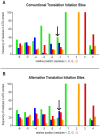
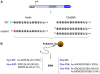
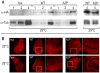
Protein modifications play a major role for most biological processes in living organisms. Amino-terminal acetylation of proteins is a common modification found throughout the tree of life: the N-terminus of a nascent polypeptide chain becomes co-translationally acetylated, often after the removal of the initiating methionine residue. While the enzymes and protein complexes involved in these processes have been extensively studied, only little is known about the biological function of such N-terminal modification events. To identify common principles of N-terminal acetylation, we analyzed the amino-terminal peptides from proteins extracted from Drosophila Kc167 cells. We detected more than 1,200 mature protein N-termini and could show that N-terminal acetylation occurs in insects with a similar frequency as in humans. As the sole true determinant for N-terminal acetylation we could extract the (X)PX rule that indicates the prevention of acetylation under all circumstances. We could show that this rule can be used to genetically engineer a protein to study the biological relevance of the presence or absence of an acetyl group, thereby generating a generic assay to probe the functional importance of N-terminal acetylation. We applied the assay by expressing mutated proteins as transgenes in cell lines and in flies. Here, we present a straightforward strategy to systematically study the functional relevance of N-terminal acetylations in cells and whole organisms. Since the (X)PX rule seems to be of general validity in lower as well as higher eukaryotes, we propose that it can be used to study the function of N-terminal acetylation in all species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Comment in
-
A simple rule for proteins to follow.PLoS Biol. 2009 Nov 3;7(11):e1000232. doi: 10.1371/journal.pbio.1000232. PLoS Biol. 2009. PMID: 20076736 Free PMC article. No abstract available.
References
-
- Bradshaw R. A, Brickey W. W, Walker K. W. N-terminal processing: the methionine aminopeptidase and N alpha-acetyl transferase families. Trends Biochem Sci. 1998;23:263–267. - PubMed
-
- Sherman F, Stewart J. W, Tsunasawa S. Methionine or not methionine at the beginning of a protein. Bioessays. 1985;3:27–31. - PubMed
-
- Boissel J. P, Kasper T. J, Bunn H. F. Cotranslational amino-terminal processing of cytosolic proteins. Cell-free expression of site-directed mutants of human hemoglobin. J Biol Chem. 1988;263:8443–8449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

