Integrin-linked kinase regulates migration and proliferation of human intestinal cells under a fibronectin-dependent mechanism
- PMID: 19885839
- PMCID: PMC2814089
- DOI: 10.1002/jcp.21963
Integrin-linked kinase regulates migration and proliferation of human intestinal cells under a fibronectin-dependent mechanism
Abstract
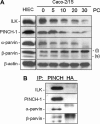
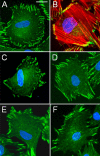
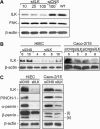
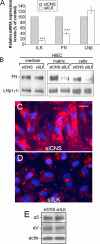
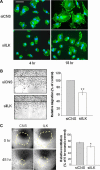
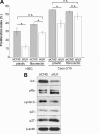
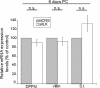
Integrin-linked kinase (ILK) plays a role in integrin signaling-mediated extracellular matrix (ECM)-cell interactions and also acts as a scaffold protein in functional focal adhesion points. In the present study, we investigated the expression and roles of ILK in human intestinal epithelial cells (IECs) in vivo and in vitro. Herein, we report that ILK and its scaffold-function interacting partners, PINCH-1, alpha-parvin, and beta-parvin, are expressed according to a decreasing gradient from the bottom of the crypt (proliferative/undifferentiated) compartment to the tip of the villus (non-proliferative/differentiated) compartment, closely following the expression pattern of the ECM/basement membrane component fibronectin. The siRNA knockdown of ILK in human IECs caused a loss of PINCH-1, alpha-parvin, and beta-parvin expression, along with a significant decrease in cell proliferation via a loss of cyclin D1 and an increase in p27 and hypophosphorylated pRb expression levels. ILK knockdown severely affected cell spreading, migration, and restitution abilities, which were shown to be directly related to a decrease in fibronectin deposition. All ILK knockdown-induced defects were rescued with exogenously deposited fibronectin. Altogether, our results indicate that ILK performs crucial roles in the control of human intestinal cell and crypt-villus axis homeostasis-especially with regard to basement membrane fibronectin deposition-as well as cell proliferation, spreading, and migration.
(c) 2009 Wiley-Liss, Inc.
Figures


References
-
- Alonso CR, Pesce CG, Kornblihtt AR. The CCAAT-binding proteins CP1 and NF-I cooperate with ATF-2 in the transcription of the fibronectin gene. J Biol Chem. 1996;271:22271–22279. - PubMed
-
- Assi K, Mills J, Owen D, Ong C, St Arnaud R, Dedhar S, Salh B. Integrin-linked kinase regulates cell proliferation and tumour growth in murine colitis-associated carcinogenesis. Gut. 2008;57:931–940. - PubMed
-
- Basson MD. In vitro evidence for matrix regulation of intestinal epithelial biology during mucosal healing. Life Sci. 2001;69:3005–3018. - PubMed
-
- Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci. 1992;102:427–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

