Telomeres and telomerase in cancer
- PMID: 19887512
- PMCID: PMC3003493
- DOI: 10.1093/carcin/bgp268
Telomeres and telomerase in cancer
Abstract
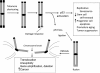
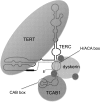
Myriad genetic and epigenetic alterations are required to drive normal cells toward malignant transformation. These somatic events commandeer many signaling pathways that cooperate to endow aspiring cancer cells with a full range of biological capabilities needed to grow, disseminate and ultimately kill its host. Cancer genomes are highly rearranged and are characterized by complex translocations and regional copy number alterations that target loci harboring cancer-relevant genes. Efforts to uncover the underlying mechanisms driving genome instability in cancer have revealed a prominent role for telomeres. Telomeres are nucleoprotein structures that protect the ends of eukaryotic chromosomes and are particularly vulnerable due to progressive shortening during each round of DNA replication and, thus, a lifetime of tissue renewal places the organism at risk for increasing chromosomal instability. Indeed, telomere erosion has been documented in aging tissues and hyperproliferative disease states-conditions strongly associated with increased cancer risk. Telomere dysfunction can produce the opposing pathophysiological states of degenerative aging or cancer with the specific outcome dictated by the integrity of DNA damage checkpoint responses. In most advanced cancers, telomerase is reactivated and serves to maintain telomere length and emerging data have also documented the capacity of telomerase to directly regulate cancer-promoting pathways. This review covers the role of telomeres and telomerase in the biology of normal tissue stem/progenitor cells and in the development of cancer.
Figures

References
-
- Palm W, et al. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. - PubMed
-
- Griffith JD, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. - PubMed
-
- van Steensel B, et al. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. - PubMed
-
- Celli GB, et al. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 2005;7:712–718. - PubMed
-
- van Steensel B, et al. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

