Crucial role of phosphatidylinositol 4-kinase IIIalpha in development of zebrafish pectoral fin is linked to phosphoinositide 3-kinase and FGF signaling
- PMID: 19887586
- PMCID: PMC2779132
- DOI: 10.1242/jcs.057646
Crucial role of phosphatidylinositol 4-kinase IIIalpha in development of zebrafish pectoral fin is linked to phosphoinositide 3-kinase and FGF signaling
Abstract
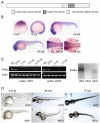
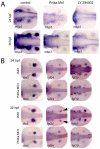
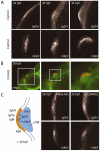
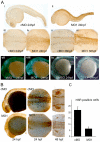
Phosphatidylinositol 4-kinases (PI4Ks) catalyze the first committed step in the synthesis of phosphoinositides, important lipid regulators of signaling and trafficking pathways. Here we cloned Pik4a, one of the zebrafish PI4K enzymes, and studied its role(s) in vertebrate development using morpholino oligonucleotide-based gene silencing in zebrafish. Downregulation of Pik4a led to multiple developmental abnormalities, affecting the brain, heart, trunk and most prominently causing loss of pectoral fins. Strikingly similar defects were caused by treatment of the developing embryos with the phosphoinositide 3-kinase (PI3K) inhibitor, LY294002. To investigate the cause of the pectoral fin developmental defect, we focused on fibroblast growth factor (FGF) signaling pathways because vertebrate limb development requires the concerted action of a series of FGF ligands. Using in situ hybridization, the pectoral fin defect was traced to disruption of the early FGF signaling loops that are crucial for the establishment of the sharp signaling center formed by the apical ectodermal ridge and the underlying mesenchyme. This, in turn caused a prominent loss of the induction of one of the mitogen-activated protein kinase (MAPK) phosphatases, Mkp3, an essential intermediate in vertebrate limb development. These changes were associated with impaired proliferation in the developing fin bud due to a loss of balance between the MAPK and PI3K branch of FGF-initiated signals. Our results identify Pik4a as an upstream partner of PI3Ks in the signaling cascade orchestrated by FGF receptors with a prominent role in forelimb development.
Figures


References
-
- Ashworth, R., Devogelaere, B., Fabes, J., Tunwell, R. E., Koh, K. R., De Smedt, H. and Patel, S. (2007). Molecular and functional characterization of inositol trisphosphate receptors during early zebrafish development. J. Biol. Chem. 282, 13984-13993. - PubMed
-
- Audhya, A. and Emr, S. D. (2002). Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593-605. - PubMed
-
- Balla, A. and Balla, T. (2006). Phosphatidylinositol 4-kinases; old enzymes with emerging functions. Trends Cell Biol. 16, 351-361. - PubMed
-
- Balla, A., Tuymetova, G., Toth, B., Szentpetery, Z., Zhao, X., Knight, Z. A., Shokat, K., Steinbach, P. J. and Balla, T. (2008). Design of drug-resistant alleles of type-III phosphatidylinositol 4-kinases using mutagenesis and molecular modeling. Biochemistry 47, 1599-1607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

