Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer
- PMID: 19887623
- PMCID: PMC2789184
- DOI: 10.1158/0008-5472.CAN-09-1499
Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer
Abstract
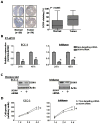
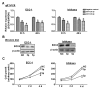
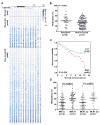
Genetic amplification, mutation, and translocation are known to play a causal role in the upregulation of an oncogene in cancer cells. Here, we report an emerging role of microRNA, the epigenetic deregulation of which may also lead to this oncogenic activation. SOX4, an oncogene belonging to the SRY-related high mobility group box family, was found to be overexpressed (P < 0.005) in endometrial tumors (n = 74) compared with uninvolved controls (n = 20). This gene is computationally predicted to be the target of a microRNA, miR-129-2. When compared with the matched endometria, the expression of miR-129-2 was lost in 27 of 31 primary endometrial tumors that also showed a concomitant gain of SOX4 expression (P < 0.001). This inverse relationship is associated with hypermethylation of the miR-129-2 CpG island, which was observed in endometrial cancer cell lines (n = 6) and 68% of 117 endometrioid endometrial tumors analyzed. Reactivation of miR-129-2 in cancer cells by pharmacologic induction of histone acetylation and DNA demethylation resulted in decreased SOX4 expression. In addition, restoration of miR-129-2 by cell transfection led to decreased SOX4 expression and reduced proliferation of cancer cells. Further analysis found a significant correlation of hypermethylated miR-129-2 with microsatellite instability and MLH1 methylation status (P < 0.001) and poor overall survival (P < 0.039) in patients. Therefore, these results imply that the aberrant expression of SOX4 is, in part, caused by epigenetic repression of miR-129-2 in endometrial cancer. Unlike the notion that promoter hypomethylation may upregulate an oncogene, we present a new paradigm in which hypermethylation-mediated silencing of a microRNA derepresses its oncogenic target in cancer cells.
Figures


References
-
- Pramoonjago P, Baras AS, Moskaluk CA. Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3 cells. Oncogene. 2006;25:5626–39. - PubMed
-
- Medina PP, Castillo SD, Blanco S, et al. The Sry-HMG box gene, SOX4, is a target of gene amplification at chromosome 6p in lung cancer. Hum Mol Genet. 2009;18:1343–52. - PubMed
-
- Liao YL, Sun YM, Chau GY, et al. Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008;27:5578–89. - PubMed
-
- Liu P, Ramachandran S, Ali Seyed M, et al. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006;66:4011–9. - PubMed
-
- Aaboe M, Birkenkamp-Demtroder K, Wiuf C, et al. SOX4 expression in bladder carcinoma: clinical aspects and in vitro functional characterization. Cancer Res. 2006;66:3434–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

