Menkes disease
- PMID: 19888294
- PMCID: PMC2987322
- DOI: 10.1038/ejhg.2009.187
Menkes disease
Abstract
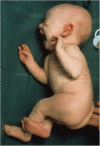
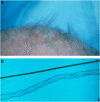
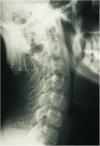
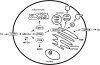
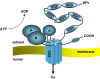
Menkes disease (MD) is a lethal multisystemic disorder of copper metabolism. Progressive neurodegeneration and connective tissue disturbances, together with the peculiar 'kinky' hair are the main manifestations. MD is inherited as an X-linked recessive trait, and as expected the vast majority of patients are males. MD occurs due to mutations in the ATP7A gene and the vast majority of ATP7A mutations are intragenic mutations or partial gene deletions. ATP7A is an energy dependent transmembrane protein, which is involved in the delivery of copper to the secreted copper enzymes and in the export of surplus copper from cells. Severely affected MD patients die usually before the third year of life. A cure for the disease does not exist, but very early copper-histidine treatment may correct some of the neurological symptoms.
Figures

References
-
- Tümer Z, Horn N.Menkes diseasein Roach ES, Miller VS (eds): Neurocutaneous Syndromes Cambridge: Cambridge University Press; 2004222–233.
-
- Horn N, Tümer Z.Menkes disease and the occipital horn syndromein Royce PM, Steinmann B (eds): Connecitive Tissue and its Heritable Disorders: Molecular, Genetic, and Medical Aspects New York: John Wiley and Sons Inc.2002. 2nd edn651–685.
-
- Lee J, Penea MMO, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;25:40253–40259. - PubMed
-
- Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:443–459. - PubMed
-
- Speisky H, Gómez M, Burgos-Bravo F, et al. Generation of superoxide radicals by copper-glutathione complexes: redox-consequences associated with their interaction with reduced glutathione. Bioorg Med Chem. 2009;17:1803–1810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

