Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices
- PMID: 19888374
- PMCID: PMC2769608
- DOI: 10.1177/193229680700100103
Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices
Abstract
Background: Continuous release of dexamethasone from PLGA microsphere/PVA hydrogel composites has been shown to suppress the inflammatory tissue reaction in response to subcutaneously implanted foreign material for a period of one month. The scope of the present work is to investigate whether suppressing the initial acute inflammatory phase with fast releasing dexamethasone-PLGA microsphere/PVA composites (that release the drug over a period of one week) would prevent the development of a foreign body reaction in response to implantation in the subcutaneous tissue using a rat model.
Methods: Dexamethasone loaded PLGA microspheres were prepared using the solvent evaporation method. In vitro release from microspheres was analyzed using USP apparatus 4 in phosphate buffered saline (PBS) at 37 degrees C. Composites were fabricated in 18G needles by freeze-thaw cycling the PVA/microsphere dispersion. The composites were implanted in the subcutaneous tissue of anesthetized rats. The pharmacodynamic effect was evaluated by histological examination of the tissue surrounding the composites at pre-determined time points.
Results: In vitro release studies showed that most of the drug entrapped in the microspheres was released within one week. At days 3 and 8, these fast releasing dexamethasone containing composites suppressed the acute phase of inflammation but did not prevent the development of an inflammatory reaction after dexamethasone was completely released from the composites. By day 30, chronic inflammation and fibrosis were observed in the tissue surrounding the drug-containing composites. On days 3 and 8, the number of inflammatory cells in the vicinity of the dexamethasone containing composites was similar to that in normal tissue. However, the number of inflammatory cells was higher in drug-containing composites as compared to drug-free composites by day 30. This was due to the inflammation being in a more advanced stage in drug-free composites where a granulomatous reaction had already developed.
Conclusion: Fast release of dexamethasone from PLGA/PVA composites did not provide long-term protection against the foreign body reaction in response to implantation. It would appear that a sustained delivery of anti-inflammatory agents such as dexamethasone is necessary to suppress inflammation throughout the implant life-time.
Keywords: biosensor; continuous release; dexamethasone; foreign body reaction; implants; localized delivery; microspheres.
Figures



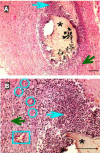

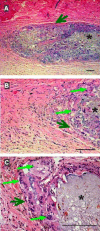


 ) with dexamethasone. Each data point represents an average of ten randomly chosen regions +/- standard deviation from each photomicrograph (n=6).
) with dexamethasone. Each data point represents an average of ten randomly chosen regions +/- standard deviation from each photomicrograph (n=6).References
-
- Gerritsen M. Problems associated with subcutaneously implanted glucose sensors. Diabetes Care. 2000;23(2):143–145. - PubMed
-
- Bolinder J, Ungerstedt U, Arner P. Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia. 1992;35(12):1177–1180. - PubMed
-
- Keck FS, Meyerhoff C, Kerner W, Siegmund T, Zier H, Pfeiffer EF. Combination of microdialysis and glucosensor permits continuous (on line) SC glucose monitoring in a patient operated device. II. Evaluation in animals. Horm Me Res. 1992;24(10):492–493. - PubMed
-
- Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–187. - PubMed
-
- Wisniewski N, Klitzman B, Miller B, Reichert WM. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57(4):513–521. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

