Oxidized LDL: diversity, patterns of recognition, and pathophysiology
- PMID: 19888833
- PMCID: PMC2877120
- DOI: 10.1089/ars.2009.2733
Oxidized LDL: diversity, patterns of recognition, and pathophysiology
Abstract
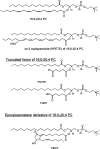
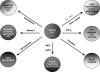
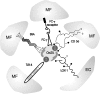
Oxidative modification of LDL is known to elicit an array of pro-atherogenic responses, but it is generally underappreciated that oxidized LDL (OxLDL) exists in multiple forms, characterized by different degrees of oxidation and different mixtures of bioactive components. The variable effects of OxLDL reported in the literature can be attributed in large part to the heterogeneous nature of the preparations employed. In this review, we first describe the various subclasses and molecular composition of OxLDL, including the variety of minimally modified LDL preparations. We then describe multiple receptors that recognize various species of OxLDL and discuss the mechanisms responsible for the recognition by specific receptors. Furthermore, we discuss the contentious issues such as the nature of OxLDL in vivo and the physiological oxidizing agents, whether oxidation of LDL is a prerequisite for atherogenesis, whether OxLDL is the major source of lipids in foam cells, whether in some cases it actually induces cholesterol depletion, and finally the Janus-like nature of OxLDL in having both pro- and anti-inflammatory effects. Lastly, we extend our review to discuss the role of LDL oxidation in diseases other than atherosclerosis, including diabetes mellitus, and several autoimmune diseases, such as lupus erythematosus, anti-phospholipid syndrome, and rheumatoid arthritis.
Figures



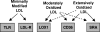

References
-
- Acton S. Rigotti A. Landschulz KT. Xu S. Hobbs HH. Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. - PubMed
-
- Acton SL. Scherer PE. Lodish HF. Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. - PubMed
-
- Adachi H. Tsujimoto M. Endothelial scavenger receptors. Prog Lipid Res. 2006;45:379–404. - PubMed
-
- Akiba S. Chiba M. Mukaida Y. Sato T. Involvement of reactive oxygen species and SP-1 in fibronectin production by oxidized LDL. Biochem Biophys Res Commun. 2003;310:491–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

