The antifungal protein PAF interferes with PKC/MPK and cAMP/PKA signalling of Aspergillus nidulans
- PMID: 19889092
- PMCID: PMC2814085
- DOI: 10.1111/j.1365-2958.2009.06936.x
The antifungal protein PAF interferes with PKC/MPK and cAMP/PKA signalling of Aspergillus nidulans
Abstract
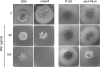
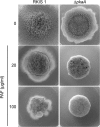
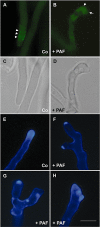
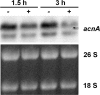
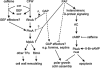
The Penicillium chrysogenum antifungal protein PAF inhibits polar growth and induces apoptosis in Aspergillus nidulans. We report here that two signalling cascades are implicated in its antifungal activity. PAF activates the cAMP/protein kinase A (Pka) signalling cascade. A pkaA deletion mutant exhibited reduced sensitivity towards PAF. This was substantiated by the use of pharmacological modulators: PAF aggravated the effect of the activator 8-Br-cAMP and partially relieved the repressive activity of caffeine. Furthermore, the Pkc/mitogen-activated protein kinase (Mpk) signalling cascade mediated basal resistance to PAF, which was independent of the small GTPase RhoA. Non-functional mutations of both genes resulted in hypersensitivity towards PAF. PAF did not increase MpkA phosphorylation or induce enzymes involved in the remodelling of the cell wall, which normally occurs in response to activators of the cell wall integrity pathway. Notably, PAF exposure resulted in actin gene repression and a deregulation of the chitin deposition at hyphal tips of A. nidulans, which offers an explanation for the morphological effects evoked by PAF and which could be attributed to the interconnection of the two signalling pathways. Thus, PAF represents an excellent tool to study signalling pathways in this model organism and to define potential fungal targets to develop new antifungals.
Figures



References
-
- Bencina M, Legisa M, Read ND. Cross-talk between cAMP and calcium signalling in Aspergillus niger. Mol Microbiol. 2005;56:268–281. - PubMed
-
- van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. Mitogenic signaling via G protein-coupled receptors. Endocrinol Rev. 1996;17:698–714. - PubMed
-
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature. 2005;3:238–250. - PubMed
-
- Bussink HJ, Osmani SA. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol Lett. 1999;173:117–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

