Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain
- PMID: 19889229
- PMCID: PMC2776013
- DOI: 10.1186/1750-1326-4-46
Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain
Abstract
Background: Variations in sortilin-related receptor (SORL1) expression and function have been implicated in Alzheimers Disease (AD). Here, to gain insights into SORL1, we evaluated SORL1 expression and splicing as a function of AD and AD neuropathology, neural gene expression and a candidate single nucleotide polymorphism (SNP).
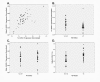
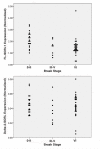
Results: To identify SORL1 splice variants, we scanned each of the 46 internal SORL1 exons in human brain RNA samples and readily found SORL1 isoforms that lack exon 2 or exon 19. Quantification in a case-control series of the more abundant isoform lacking exon 2 (delta-2-SORL1), as well as the "full-length" SORL1 (FL-SORL1) isoform containing exon 2 showed that expression of FL-SORL1 was reduced in AD individuals. Moreover, FL-SORL1 was reduced in cognitively intact individuals with significant AD-like neuropathology. In contrast, the expression of the delta-2-SORL1 isoform was similar in AD and non-AD brains. The expression of FL-SORL1 was significantly associated with synaptophysin expression while delta-2-SORL1 was modestly enriched in white matter. Lastly, FL-SORL1 expression was associated with rs661057, a SORL1 intron one SNP that has been associated with AD risk. A linear regression analysis found that rs661057, synaptophysin expression and AD neuropathology were each associated with FL-SORL1 expression.
Conclusion: These results confirm that FL-SORL1 expression declines in AD and with AD-associated neuropathology, suggest that FL-SORL1 declines in cognitively-intact individuals with AD-associated neuropathology, identify a novel SORL1 splice variant that is expressed similarly in AD and non-AD individuals, and provide evidence that an AD-associated SNP is associated with SORL1 expression. Overall, these results contribute to our understanding of SORL1 expression in the human brain.
Figures




References
-
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102:13461–6. doi: 10.1073/pnas.0503689102. - DOI - PMC - PubMed
-
- Dodson SE, Andersen OM, Karmali V, Fritz JJ, Cheng D, Peng J, Levey AI, Willnow TE, Lah JJ. Loss of LR11/sorLA enhances early pathology in a mouse model of amyloidosis: Evidence for a proximal role in Alzheimer's disease. J Neurosci. 2008;28:12877–86. doi: 10.1523/JNEUROSCI.4582-08.2008. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

