Plasmodium falciparum exposure in utero, maternal age and parity influence the innate activation of foetal antigen presenting cells
- PMID: 19889240
- PMCID: PMC2780449
- DOI: 10.1186/1475-2875-8-251
Plasmodium falciparum exposure in utero, maternal age and parity influence the innate activation of foetal antigen presenting cells
Abstract
Background: Malaria in pregnancy is associated with immunological abnormalities in the newborns, such as hampered T-helper 1 responses and increased T-regulatory responses, while the effect of maternal Plasmodium falciparum infection on foetal innate immunity is still controversial.
Materials and methods: The immunophenotype and cytokine release by dendritic cells (DC) and monocytes were evaluated in cord blood from 59 Beninese women with or without malaria infection by using flow cytometry.
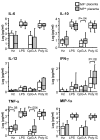
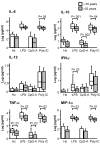
Results: Accumulation of malaria pigment in placenta was associated with a partial maturation of cord blood myeloid and plasmacytoid DC, as reflected by an up-regulated expression of the major histocompatibility complex class II molecules, but not CD86 molecules. Cells of newborns of mothers with malaria pigment in their placenta also exhibited significantly increased cytokine responses upon TLR9 stimulation. In addition, maternal age and parity influenced the absolute numbers and activation status of cord blood antigen-presenting cells. Lastly, maternal age, but not parity, influenced TLR3, 4 and 9 responses in cord blood cells.
Discussion: Our findings support the view that placental parasitization, as indicated by the presence of malaria pigment in placental leukocytes, is significantly associated with partial maturation of different DC subsets and also to slightly increased responses to TLR9 ligand in cord blood. Additionally, other factors, such as maternal age and parity should be taken into consideration when analysing foetal/neonatal innate immune responses.
Conclusion: These data advocate a possible mechanism by which PAM may modulate foetal/neonatal innate immunity.
Figures




References
-
- Elliott SR, Spurck TP, Dodin JM, Maier AG, Voss TS, Yosaatmadja F, Payne PD, McFadden GI, Cowman AF, Rogerson SJ, Schofield L, Brown GV. Inhibition of Dendritic Cell Maturation by Malaria Is Dose Dependent and Does Not Require Plasmodium falciparum Erythrocyte Membrane Protein 1. Infect Immun. 2007;75:3621–3632. doi: 10.1128/IAI.00095-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

