Markers of fitness in a successful enzyme superfamily
- PMID: 19889535
- PMCID: PMC2787785
- DOI: 10.1016/j.sbi.2009.09.008
Markers of fitness in a successful enzyme superfamily
Abstract
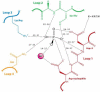
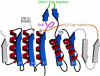
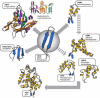
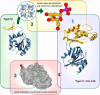
Haloalkanoic acid dehalogenase (HAD) superfamily members serve as the predominant catalysts of metabolic phosphate ester hydrolysis in all three superkingdoms of life. Collectively, the known structural, bioinformatic, and mechanistic data offer a glimpse of the variety of HAD enzymes that have evolved in the service of metabolic expansion. Factors that have contributed to superfamily dominance include a chemically versatile nucleophile, stability of the core superfold, structural modularity of the chemistry and specificity domains, conformational coupling conferred by the topology of the inserted specificity elements, and retention of a conserved mold for stabilization of the trigonal bipyramidal transition state.
Figures



References
-
- Wolfenden R, Snider MJ. The depth of chemical time and the power of enzymes as catalysts. Acc Chem Res. 2001;34(12):938–45. - PubMed
-
- Collet JF, van Schaftingen E, Stroobant V. A new family of phosphotransferases related to P-type ATPases. Trends Biochem Sci. 1998;23(8):284. - PubMed
-
- Koonin EV, Tatusov RL. Computer Analysis of Bacterial Haloacid Dehalogenases Defines a Large Superfamily of Hydrolases with Diverse Specificity: Application of an Iterative Approach to Database Search. Journal of Molecular Biology. 1994;244(1):125–32. - PubMed
-
- Aravind L, Galperin MY, Koonin EV. The catalytic domain of the P-type ATPase has the haloacid dehalogenase fold. Trends Biochem Sci. 1998;23(4):127–9. - PubMed
-
- Collet J-F, Gerin I, Rider MH, Veiga-da-Cunha M, Van Schaftingen E. Human - 3-phosphoserine phosphatase: sequence, expression and evidence for a phosphoenzyme intermediate. FEBS Letters. 1997;408(3):281–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

