Inherent anti-amyloidogenic activity of human immunoglobulin gamma heavy chains
- PMID: 19889627
- PMCID: PMC2801233
- DOI: 10.1074/jbc.M109.044321
Inherent anti-amyloidogenic activity of human immunoglobulin gamma heavy chains
Abstract
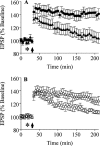
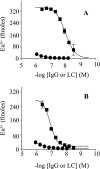
We have previously shown that a subpopulation of naturally occurring human IgGs were cross-reactive against conformational epitopes on pathologic aggregates of Abeta, a peptide that forms amyloid fibrils in the brains of patients with Alzheimer disease, inhibited amyloid fibril growth, and dissociated amyloid in vivo. Here, we describe similar anti-amyloidogenic activity that is a general property of free human Ig gamma heavy chains. A gamma(1) heavy chain, F1, had nanomolar binding to an amyloid fibril-related conformational epitope on synthetic oligomers and fibrils as well as on amyloid-laden tissue sections. F1 did not bind to native Abeta monomers, further indicating the conformational nature of its binding site. The inherent anti-amyloidogenic activity of Ig gamma heavy chains was demonstrated by nanomolar amyloid fibril and oligomer binding by polyclonal and monoclonal human heavy chains that were isolated from inert or weakly reactive antibodies. Most importantly, the F1 heavy chain prevented in vitro fibril growth and reduced in vivo soluble Abeta oligomer-induced impairment of rodent hippocampal long term potentiation, a cellular mechanism of learning and memory. These findings demonstrate that free human Ig gamma heavy chains comprise a novel class of molecules for developing potential therapeutics for Alzheimer disease and other amyloid disorders. Moreover, establishing the molecular basis for heavy chain-amyloidogenic conformer interactions should advance understanding on the types of interactions that these pathologic assemblies have with biological molecules.
Figures



References
-
- Stefani M. (2004) Biochim. Biophys. Acta 1739, 5–25 - PubMed
-
- Goedert M., Spillantini M. G. (2006) Science 314, 777–781 - PubMed
-
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B., Selkoe D. J. (1992) Nature 359, 322–325 - PubMed
-
- Hardy J., Selkoe D. J. (2002) Science 297, 353–356 - PubMed
-
- Walsh D. M., Selkoe D. J. (2007) J. Neurochem. 101, 1172–1184 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

