Modification of small hepatitis delta virus antigen by SUMO protein
- PMID: 19889771
- PMCID: PMC2798338
- DOI: 10.1128/JVI.01034-09
Modification of small hepatitis delta virus antigen by SUMO protein
Abstract
Hepatitis delta antigen (HDAg) is a nuclear protein that is intimately involved in hepatitis delta virus (HDV) RNA replication. HDAg consists of two protein species, the small form (S-HDAg) and the large form (L-HDAg). Previous studies have shown that posttranslational modifications of S-HDAg, such as phosphorylation, acetylation, and methylation, can modulate HDV RNA replication. In this study, we show that S-HDAg is a small ubiquitin-like modifier 1 (SUMO1) target protein. Mapping data showed that multiple lysine residues are SUMO1 acceptors within S-HDAg. Using a genetic fusion strategy, we found that conjugation of SUMO1 to S-HDAg selectively enhanced HDV genomic RNA and mRNA synthesis but not antigenomic RNA synthesis. This result supports our previous proposition that the cellular machinery involved in the synthesis of HDV antigenomic RNA is different from that for genomic RNA synthesis and mRNA transcription, requiring different modified forms of S-HDAg. Sumoylation represents a new type of modification for HDAg.
Figures

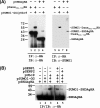






References
-
- Boddy, M. N., K. Howe, L. D. Etkin, E. Solomon, and P. S. Freemont. 1996. PIC 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene 13:971-982. - PubMed
-
- Carter, S., and K. H. Vousden. 2008. p53-Ubl fusions as models of ubiquitination, sumoylation and neddylation of p53. Cell Cycle 7:2519-2528. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

