Protective memory responses are modulated by priming events prior to challenge
- PMID: 19889782
- PMCID: PMC2798378
- DOI: 10.1128/JVI.01535-09
Protective memory responses are modulated by priming events prior to challenge
Abstract
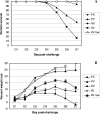
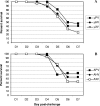
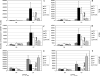
Human infections with highly pathogenic H5N1 avian influenza A viruses in the last decade have legitimized fears of a long-predicted pandemic. We thus investigated the response to secondary infections with an engineered, but still highly virulent, H5N1 influenza A virus in the C57BL/6 mouse model. Mice primed with the H1N1 A/Puerto Rico/8/34 (PR8) virus were partially protected from lethality following respiratory infection with the modified H5N1 virus A/Vietnam/1203/04 (DeltaVn1203). In contrast, those that had been comparably exposed to the HKx31 (H3N2) virus succumbed to the DeltaVn1203 challenge, despite similarities in viral replication, weight loss, and secondary CD8(+)-T-cell response characteristics. All three viruses share the internal genes of PR8 that are known to stimulate protective CD8(+)-T-cell-mediated immunity. This differential survival of PR8- and HKx31-primed mice was also apparent for antibody-deficient mice challenged with the DeltaVn1203 virus. The relative protection afforded by PR8 priming was abrogated in tumor necrosis factor-deficient (TNF(-/-)) mice, although lung fluids from the B6 HKx31-primed mice contained more TNF early after challenge. These data demonstrate that the nature of the primary infection can influence pathological outcomes following virulent influenza virus challenge, although the effect is not clearly correlated with classical measures of CD8(+)-T-cell-mediated immunity.
Figures



References
-
- Aldridge, J. R., Jr., C. E. Moseley, D. A. Boltz, N. J. Negovetich, C. Reynolds, J. Franks, S. A. Brown, P. C. Doherty, R. G. Webster, and P. G. Thomas. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza infection. Proc. Natl. Acad. Sci. U. S. A. 106:5306-5311. - PMC - PubMed
-
- Barry, J. M. 2004. The great influenza. The epic story of the deadliest plague in history. Viking, New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

