Age-dependent preferential dense-core vesicle exocytosis in neuroendocrine cells revealed by newly developed monomeric fluorescent timer protein
- PMID: 19889833
- PMCID: PMC2801723
- DOI: 10.1091/mbc.e09-08-0722
Age-dependent preferential dense-core vesicle exocytosis in neuroendocrine cells revealed by newly developed monomeric fluorescent timer protein
Abstract
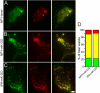
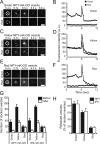
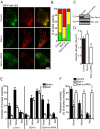
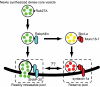
Although it is evident that only a few secretory vesicles accumulating in neuroendocrine cells are qualified to fuse with the plasma membrane and release their contents to the extracellular space, the molecular mechanisms that regulate their exocytosis are poorly understood. For example, it has been controversial whether secretory vesicles are exocytosed randomly or preferentially according to their age. Using a newly developed protein-based fluorescent timer, monomeric Kusabira Green Orange (mK-GO), which changes color with a predictable time course, here we show that small GTPase Rab27A effectors regulate age-dependent exocytosis of secretory vesicles in PC12 cells. When the vesicles were labeled with mK-GO-tagged neuropeptide Y or tissue-type plasminogen activator, punctate structures with green or red fluorescence were observed. Application of high [K(+)] stimulation induced exocytosis of new (green) fluorescent secretory vesicles but not of old (red) vesicles. Overexpression or depletion of rabphilin and synaptotagmin-like protein4-a (Slp4-a), which regulate exocytosis positively and negatively, respectively, disturbed the age-dependent exocytosis of the secretory vesicles in different manners. Our results suggest that coordinate functions of the two effectors of Rab27A, rabphilin and Slp4-a, are required for regulated secretory pathway.
Figures


References
-
- Baldini G., Martelli A. M., Tabellini G., Horn C., Machaca K., Narducci P., Baldini G. Rabphilin localizes with the cell actin cytoskeleton and stimulates association of granules with F-actin cross-linked by α-actinin. J. Biol. Chem. 2005;280:34974–34984. - PubMed
-
- Cheviet S., Waselle L., Regazzi R. Noc-king out exocrine and endocrine secretion. Trends Cell Biol. 2004;14:525–528. - PubMed
-
- Dannies P. S. Mechanisms for storage of prolactin and growth hormone in secretory granules. Mol. Genet. Metab. 2002;76:6–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

