Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations
- PMID: 19889834
- PMCID: PMC2801714
- DOI: 10.1091/mbc.e09-07-0597
Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations
Abstract
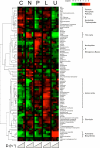
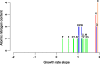
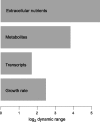
Microbes tailor their growth rate to nutrient availability. Here, we measured, using liquid chromatography-mass spectrometry, >100 intracellular metabolites in steady-state cultures of Saccharomyces cerevisiae growing at five different rates and in each of five different limiting nutrients. In contrast to gene transcripts, where approximately 25% correlated with growth rate irrespective of the nature of the limiting nutrient, metabolite concentrations were highly sensitive to the limiting nutrient's identity. Nitrogen (ammonium) and carbon (glucose) limitation were characterized by low intracellular amino acid and high nucleotide levels, whereas phosphorus (phosphate) limitation resulted in the converse. Low adenylate energy charge was found selectively in phosphorus limitation, suggesting the energy charge may actually measure phosphorus availability. Particularly strong concentration responses occurred in metabolites closely linked to the limiting nutrient, e.g., glutamine in nitrogen limitation, ATP in phosphorus limitation, and pyruvate in carbon limitation. A simple but physically realistic model involving the availability of these metabolites was adequate to account for cellular growth rate. The complete data can be accessed at the interactive website http://growthrate.princeton.edu/metabolome.
Figures




References
-
- Abbott D. A., van den Brink J., Minneboo I. M., Pronk J. T., van Maris A. J. Anaerobic homolactate fermentation with Saccharomyces cerevisiae results in depletion of ATP and impaired metabolic activity. FEMS Yeast Res. 2009;9:349–357. - PubMed
-
- Auesukaree C., Tochio H., Shirakawa M., Kaneko Y., Harashima S. Plc1p, Arg82p, and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polyphosphate accumulation in Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:25127–25133. - PubMed
-
- Avigad G. Stimulation of yeast phosphofructokinase activity by fructose 2,6-bisphosphate. Biochemical and biophysical research communications. 1981;102:7. - PubMed
-
- Bajad S. U., Lu W., Kimball E. H., Yuan J., Peterson C., Rabinowitz J. D. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography—tandem mass spectrometry. J. Chromatogr. A. 2006;1125:76–88. - PubMed
-
- Barford J. P., Hall R. J. Investigation of the significance of a carbon and redox balance to the measurement of gaseous metabolism of Saccharomyces cerevisiae. Biotechnol. Bioeng. 1979;21:609–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

