Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure
- PMID: 19889963
- PMCID: PMC2822493
- DOI: 10.1152/ajpcell.00336.2009
Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure
Abstract
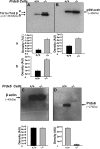
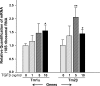
Oxidative stress is one of the causative factors in progression and etiology of age-related cataract. Peroxiredoxin 6 (Prdx6), a savior for cells from internal or external environmental stresses, plays a role in cellular signaling by detoxifying reactive oxygen species (ROS) and thereby controlling gene regulation. Using targeted inactivation of the Prdx6 gene, we show that Prdx6-deficient lens epithelial cells (LECs) are more vulnerable to UV-triggered cell death, a major cause of skin disorders including cataractogenesis, and these cells display abnormal protein profiles. PRDX6-depleted LECs showed phenotypic changes and formed lentoid body, a characteristic of terminal cell differentiation and epithelial-mesenchymal transition. Prdx6(-/-) LECs exposed to UV-B showed higher ROS expression and were prone to apoptosis compared with wild-type LECs, underscoring a protective role for Prdx6. Comparative proteomic analysis using fluorescence-based difference gel electrophoresis along with mass spectrometry and database searching revealed a total of 13 proteins that were differentially expressed in Prdx6(-/-) cells. Six proteins were upregulated, whereas expression of seven proteins was decreased compared with Prdx6(+/+) LECs. Among the cytoskeleton-associated proteins that were highly expressed in Prdx6-deficient LECs was tropomyosin (Tm)2beta. Protein blot and real-time PCR validated dramatic increase of Tm2beta and Tm1alpha expression in these cells. Importantly, Prdx6(+/+) LECs showed a similar pattern of Tm2beta protein expression after transforming growth factor (TGF)-beta or H(2)O(2) treatment. An extrinsic supply of PRDX6 could restore Tm2beta expression, demonstrating that PRDX6 may attenuate adverse signaling in cells and thereby maintain cellular homeostasis. Exploring redox-proteomics (Prdx6(-/-)) and characterization and identification of abnormally expressed proteins and their attenuation by PRDX6 delivery should provide a basis for development of novel therapeutic interventions to postpone ROS-mediated abnormal signaling deleterious to cells or tissues.
Figures








References
-
- Andley UP, Clark BA. The effects of near-UV radiation on human lens beta-crystallins: protein structural changes and the production of O2- and H2O2. Photochem Photobiol 50: 97–105, 1989 - PubMed
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 116: 217–224, 2003 - PubMed
-
- Arita T, Lin LR, Reddy VN. Differentiation of human lens epithelial cells in tissue culture. Exp Eye Res 47: 905–910, 1988 - PubMed
-
- Arita T, Lin LR, Susan SR, Reddy VN. Enhancement of differentiation of human lens epithelium in tissue culture by changes in cell-substrate adhesion. Invest Ophthalmol Vis Sci 31: 2395–2404, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

