Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling
- PMID: 19889983
- PMCID: PMC2780750
- DOI: 10.1073/pnas.0905884106
Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling
Abstract
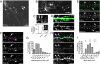
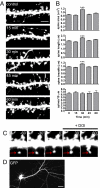
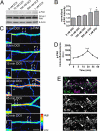
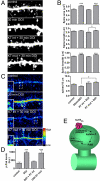
The 5-HT(2A) serotonin receptor is the most abundant serotonin receptor subtype in the cortex and is predominantly expressed in pyramidal neurons. The 5-HT(2A) receptor is a target of several hallucinogens, antipsychotics, anxiolytics, and antidepressants, and it has been associated with several psychiatric disorders, conditions that are also associated with aberrations in dendritic spine morphogenesis. However, the role of 5-HT(2A) receptors in regulating dendritic spine morphogenesis in cortical neurons is unknown. Here we show that the 5-HT(2A) receptor is present in a subset of spines, in addition to dendritic shafts. It colocalizes with PSD-95 and with multiple PDZ protein-1 (MUPP1) in a subset of dendritic spines of rat cortical pyramidal neurons. MUPP1 is enriched in postsynaptic density (PSD) fractions, is targeted to spines in pyramidal neurons, and enhances the localization of 5-HT(2A) receptors to the cell periphery. 5-HT(2A) receptor activation by the 5-HT(2) receptor agonist DOI induced a transient increase in dendritic spine size, as well as phosphorylation of p21-activated kinase (PAK) in cultured cortical neurons. PAK is a downstream target of the neuronal Rac guanine nucleotide exchange factor (RacGEF) kalirin-7 that is important for spine remodeling. Kalirin-7 regulates dendritic spine morphogenesis in neurons but its role in neuromodulator signaling has not been investigated. We show that peptide interference that prevents the localization of kalirin-7 to the postsynaptic density disrupts DOI-induced PAK phosphorylation and spine morphogenesis. These results suggest a potential role for serotonin signaling in modulating spine morphology and kalirin-7's function at cortical synapses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
-
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: Emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. - PubMed
-
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. - PubMed
-
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

