Inositol 1,4,5-trisphosphate 3-kinase a functions as a scaffold for synaptic Rac signaling
- PMID: 19890013
- PMCID: PMC2818627
- DOI: 10.1523/JNEUROSCI.2483-09.2009
Inositol 1,4,5-trisphosphate 3-kinase a functions as a scaffold for synaptic Rac signaling
Abstract
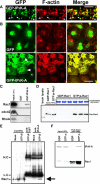
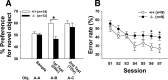
Activity-dependent alterations of synaptic contacts are crucial for synaptic plasticity. The formation of new dendritic spines and synapses is known to require actin cytoskeletal reorganization specifically during neural activation phases. Yet the site-specific and time-dependent mechanisms modulating actin dynamics in mature neurons are not well understood. In this study, we show that actin dynamics in spines is regulated by a Rac anchoring and targeting function of inositol 1,4,5-trisphosphate 3-kinase A (IP(3)K-A), independent of its kinase activity. On neural activation, IP(3)K-A bound directly to activated Rac1 and recruited it to the actin cytoskeleton in the postsynaptic area. This focal targeting of activated Rac1 induced spine formation through actin dynamics downstream of Rac signaling. Consistent with the scaffolding role of IP(3)K-A, IP(3)K-A knock-out mice exhibited defects in accumulation of PAK1 by long-term potentiation-inducing stimulation. This deficiency resulted in a reduction in the reorganization of actin cytoskeletal structures in the synaptic area of dentate gyrus. Moreover, IP(3)K-A knock-out mice showed deficits of synaptic plasticity in perforant path and in hippocampal-dependent memory performances. These data support a novel model in which IP(3)K-A is critical for the spatial and temporal regulation of spine actin remodeling, synaptic plasticity, and learning and memory via an activity-dependent Rac scaffolding mechanism.
Figures






References
-
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. - PubMed
-
- Choi KY, Kim HK, Lee SY, Moon KH, Sim SS, Kim JW, Chung HK, Rhee SG. Molecular cloning and expression of a complementary DNA for inositol 1,4,5-trisphosphate 3-kinase. Science. 1990;248:64–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
