NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation
- PMID: 19890047
- PMCID: PMC2786082
- DOI: 10.4049/jimmunol.0900582
NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation
Abstract
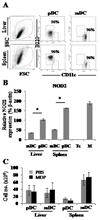
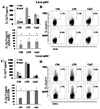
The nucleotide-binding oligomerization domain (NOD)2/CARD15 protein, which senses muramyl dipeptide (MDP), a product of bacterial peptidoglycan, appears to play an important role in regulating intestinal immunity. Although the liver is exposed to gut-derived MDP, the influence of NOD2 ligation on hepatic APC, in particular dendritic cells (DC), is unknown. Freshly isolated mouse liver and spleen plasmacytoid (p)DC expressed higher levels of NOD2 message than conventional myeloid (m)DC. Following MDP stimulation in vivo, liver pDC, but not mDC, up-regulated expression of IFN regulatory factor 4 (IRF-4), a negative regulator of TLR signaling, and induced less allogeneic T cell proliferation and IFN-gamma production. The adoptive transfer of liver pDC from MDP-treated mice failed to prime allogeneic T cells in vivo. By contrast, splenic DC IRF-4 levels and T cell stimulatory activity remained unchanged. Liver pDC from MDP-stimulated mice also displayed greater IkappaBalpha, cell surface B7-H1, and B7-H1 relative to CD86 than control liver pDC. No similar effects were observed for liver mDC or spleen DC. Absence of B7-H1 on liver pDC reversed the inhibitory effect of MDP. After ex vivo stimulation with LPS or CpG, liver pDC but not mDC from MDP-treated animals secreted less IL-12p70, IL-6, and TNF-alpha and induced weaker allogeneic T cell proliferation than those from controls. Moreover, CpG-stimulated liver pDC from MDP-treated mice secreted less IFN-alpha than their splenic counterparts, and systemic levels of IFN-alpha were reduced in MDP-treated animals after CpG administration. These findings suggest that differential effects of NOD2 ligation on liver pDC may play a role in regulating hepatic innate and adaptive immunity.
Figures







References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
-
- Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. - PubMed
-
- Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

