Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes
- PMID: 19890056
- PMCID: PMC2921715
- DOI: 10.4049/jimmunol.0901906
Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes
Abstract
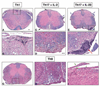
Experimental autoimmune encephalomyelitis (EAE) is a model of human multiple sclerosis induced by autoreactive Th cells that mediate tissue inflammation and demyelination in the CNS. Initially, IFN-gamma-producing Th1 cells and, more recently, IL-17-producing Th17 cells with specificity for myelin Ags have been implicated in EAE induction, but whether Th17 cells are encephalitogenic has been controversial. Moreover, a new effector T cell subset, Th9 cells, has been identified; however, the ability of this T cell subset to induce EAE has not been investigated. Here, we have developed protocols to generate myelin oligodendrocyte glycoprotein-specific Th17, Th1, Th2, and Th9 cells in vitro, so that we could directly compare and characterize the encephalitogenic activity of each of these subsets upon adoptive transfer. We show that myelin oligodendrocyte glycoprotein-specific Th1, Th17, and Th9 cells but not Th2 cells induce EAE upon adoptive transfer. Importantly, each T cell subset induced disease with a different pathological phenotype. These data demonstrate that different effector T cell subsets with specificity for myelin Ags can induce CNS autoimmunity and that the pathological heterogeneity in multiple sclerosis lesions might in part be due to multiple distinct myelin-reactive effector T cells.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures







References
-
- Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS030843/NS/NINDS NIH HHS/United States
- R01NS045937/NS/NINDS NIH HHS/United States
- R01 NS059996/NS/NINDS NIH HHS/United States
- R37 NS030843/NS/NINDS NIH HHS/United States
- R01 NS035685/NS/NINDS NIH HHS/United States
- R01A1044880/PHS HHS/United States
- P01A1039671/PHS HHS/United States
- R29 NS030843/NS/NINDS NIH HHS/United States
- R37NS030843/NS/NINDS NIH HHS/United States
- P01 NS038037/NS/NINDS NIH HHS/United States
- R01 NS045937/NS/NINDS NIH HHS/United States
- 1R01NS059996/NS/NINDS NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R01NS035685/NS/NINDS NIH HHS/United States
- P01NS038037/NS/NINDS NIH HHS/United States
- R01 AI044880/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

