Safety and pharmacokinetics of repeat-dose micafungin in young infants
- PMID: 19890251
- PMCID: PMC2824925
- DOI: 10.1038/clpt.2009.200
Safety and pharmacokinetics of repeat-dose micafungin in young infants
Abstract
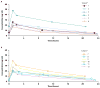
Given the risk of central nervous system infection, relatively high weight-based echinocandin dosages may be required for the successful treatment of invasive candidiasis and candidemia in young infants. This open-label study assessed the safety and pharmacokinetics (PK) of micafungin in 13 young infants (>48 h and <120 days of life) with suspected candidemia or invasive candidiasis. Infants of body weight > or =1,000 and <1,000 g received 7 and 10 mg/kg/day, respectively, for a minimum of 4-5 days. In the 7-mg/kg/day group, the mean baseline weight and gestational age were 2,101 g and 30 weeks, respectively; in the 10-mg/kg/day group, they were 688 g and 25 weeks, respectively. The median pharmacokinetic values for the 7- and 10-mg/kg/day groups, respectively, were as follows: area under the concentration-time curve from 0 to 24 h (AUC(0-24)), 258.1 and 291.2 microg x h/ml; clearance at steady state adjusted for body weight, 0.45 and 0.57 ml/min/kg; maximum plasma concentration, 23.3 and 24.9 micro g/ml; and volume of distribution at steady state adjusted for body weight, 341.4 and 542.8 ml/kg. No deaths or discontinuations from treatment occurred. These data suggest that micafungin dosages of 7 and 10 mg/kg/day are well tolerated and provide exposure levels that have been shown (in animal models) to be adequate for central nervous system coverage.
Conflict of interest statement
Authors declare the following potential conflicts of interest:
DK Benjamin Jr. receives support from the United States Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the non-profit organization Thrasher Research Foundation for his work in neonatal candidiasis (
L Castro, WW Hope, TJ Walsh, and D Kaufman do not declare any potential conflicts of interest.
Figures
References
-
- Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112:634–40. - PubMed
-
- Benjamin DK, Jr, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. - PubMed
-
- Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–6. - PubMed
-
- Stoll BJ, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. - PubMed
-
- Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ. R.H. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112:543–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical