The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis
- PMID: 19890268
- PMCID: PMC3428627
- DOI: 10.1038/mi.2009.124
The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis
Abstract
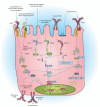
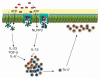
The mucosal surfaces of the gastrointestinal tract are continually exposed to an enormous antigenic load of microbial and dietary origin, yet homeostasis is maintained. Pattern recognition molecules (PRMs) have a key role in maintaining the integrity of the epithelial barrier and in promoting maturation of the mucosal immune system. Commensal bacteria modulate the expression of a broad range of genes involved in maintaining epithelial integrity, inflammatory responses, and production of antimicrobial peptides. Mice deficient in PRMs can develop intestinal inflammation, which is dependent on the microbiota, and in humans, PRM polymorphisms are associated with exacerbated inflammatory bowel disease. Innate immune responses and epithelial barrier function are regulated by PRM-induced signaling at multiple levels, from the selective expression of receptors on mucosal cells or compartments to the expression of negative regulators. Here, we describe recent advances in our understanding of innate signaling pathways, particularly by Toll-like receptors and nucleotide-binding domain and leucine-rich repeat containing receptors at mucosal surfaces.
Figures
Similar articles
-
Expression and functional importance of innate immune receptors by intestinal epithelial cells.Cell Mol Life Sci. 2011 Nov;68(22):3661-73. doi: 10.1007/s00018-011-0829-9. Epub 2011 Oct 8. Cell Mol Life Sci. 2011. PMID: 21984599 Free PMC article. Review.
-
Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases.Int J Mol Sci. 2021 Dec 13;22(24):13397. doi: 10.3390/ijms222413397. Int J Mol Sci. 2021. PMID: 34948194 Free PMC article. Review.
-
The role of innate immunity receptors in the pathogenesis of inflammatory bowel disease.Mediators Inflamm. 2015;2015:936193. doi: 10.1155/2015/936193. Epub 2015 Mar 4. Mediators Inflamm. 2015. PMID: 25821356 Free PMC article. Review.
-
Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease.Int J Mol Sci. 2021 Jul 16;22(14):7618. doi: 10.3390/ijms22147618. Int J Mol Sci. 2021. PMID: 34299236 Free PMC article. Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair.Gastroenterology. 2016 Oct;151(4):616-32. doi: 10.1053/j.gastro.2016.07.008. Epub 2016 Jul 18. Gastroenterology. 2016. PMID: 27436072 Free PMC article. Review.
-
The role of the gut microbiota in health and cardiovascular diseases.Mol Biomed. 2022 Oct 11;3(1):30. doi: 10.1186/s43556-022-00091-2. Mol Biomed. 2022. PMID: 36219347 Free PMC article. Review.
-
Expression of Toll-Like Receptors 2, 4 and 5 in Relation to Gut Microbiota in Colon Neoplasm Patients with and without Inflammatory Bowel Disease.Avicenna J Med Biotechnol. 2022 Jul-Sep;14(3):188-195. doi: 10.18502/ajmb.v14i3.9825. Avicenna J Med Biotechnol. 2022. PMID: 36061133 Free PMC article.
-
Euglena Gracilis and β-Glucan Paramylon Induce Ca2+ Signaling in Intestinal Tract Epithelial, Immune, and Neural Cells.Nutrients. 2020 Jul 30;12(8):2293. doi: 10.3390/nu12082293. Nutrients. 2020. PMID: 32751743 Free PMC article.
-
Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities.Cancers (Basel). 2023 Jan 30;15(3):866. doi: 10.3390/cancers15030866. Cancers (Basel). 2023. PMID: 36765824 Free PMC article. Review.
References
-
- Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends. Immunol. 2006;27:352–357. - PubMed
-
- Rakoff-Nahoum S, Pagina J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microfl ora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. - PubMed
-
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host–microbial relationships in the intestine. Science. 2001;291:881–884. - PubMed
-
- Macpherson AJ, Slack E, Geuking MB, McCoy KD. The mucosal firewalls against commensal intestinal microbes. Semin. Immunopathol. 2009;31:145–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

