Fluorescence Quenching of CdTe Nanocrystals by Bound Gold Nanoparticles in Aqueous Solution
- PMID: 19890452
- PMCID: PMC2772157
- DOI: 10.1007/s11468-007-9047-6
Fluorescence Quenching of CdTe Nanocrystals by Bound Gold Nanoparticles in Aqueous Solution
Abstract
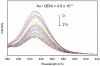
Water-soluble gold nanoparticles with an average diameter of 5 nm were prepared with carboxylic acid terminated thiol ligands. These ligands contain zero to eight methylene moieties. CdTe nanocrystals with an average diameter of 5 nm were synthesized with aminoethanethiol capping. These nanocrystals displayed characteristic absorption and emission spectra of quantum dots. The amine terminated CdTe nanocrystals and carboxylic-acid-terminated gold nanoparticles were conjugated in aqueous solution at pH 5.0 by electrostatic interaction, and the conjugation was monitored with fluorescence spectroscopy. The CdTe nanocrystals were significantly quenched upon binding with gold nanoparticles. The quenching efficiency was affected by both the concentration of gold nanoparticles in the complex and the length of spacer between the CdTe nanocrystal and Au nanoparticle. The observed quenching was explained using Förster resonance energy transfer (FRET) mechanism, and the Förster distance was estimated to be 3.8 nm between the donor-acceptor pair.
Figures







References
-
- Thomas KG, Kamat PV. Chromophore-functionalized gold nanoparticles. Acc Chem Res. 2003;36:888–898. - PubMed
-
- Kamat PV. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J Phys Chem B. 2002;106:7729–7744.
-
- Emory SR, Haskins WE, Nie S. Direct observation of size-dependent optical enhancement in single metal nanoparticles. J Am Chem Soc. 1998;120:8009.
-
- Goodrich GP, Helfrich MR, Overberg JJ, Keating CD. Effect of macromolecular crowding on DNA: Au nanoparticle bioconjugate assembly. Langmuir. 2004;20:10246–10251. - PubMed
-
- Josephson L, Kircher MF, Mahmood U, Tang Y, Weissleder R. Near-infrared fluorescent nanoparticles as combined MR/optical imaging probes. Bioconjugate Chem. 2002;13:554–560. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
