Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study
- PMID: 19890959
- PMCID: PMC2916680
- DOI: 10.1002/cncr.24714
Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study
Abstract
Background: Bendamustine hydrochloride is a novel alkylating agent. In this multicenter study, the authors evaluated the efficacy and toxicity of single-agent bendamustine in patients with rituximab-refractory, indolent B-cell lymphoma.
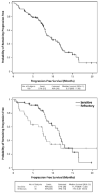
Methods: Eligible patients (N = 100, ages 31-84 years) received bendamustine at a dose of 120 mg/m(2) by intravenous infusion on Days 1 and 2 every 21 days for 6 to 8 cycles. Histologies included follicular (62%), small lymphocytic (21%), and marginal zone (16%) lymphomas. Patients had received a median of 2 previous regimens (range, 0-6 previous regimens), and 36%were refractory to their most recent chemotherapy regimen. Primary endpoints included overall response rate (ORR) and duration of response (DOR). Secondary endpoints were safety and progression-free survival (PFS).
Results: An ORR of 75% (a 14% complete response rate, a 3% unconfirmed complete response rate, and a 58% partial response rate) was observed. The median DOR was 9.2 months, and median PFS was 9.3 months. Six deaths were considered to be possibly treatment related. Grade 3 or 4 (determined using National Cancer Institute Common Toxicity Criteria [version 3.0.19]. reversible hematologic toxicities included neutropenia (61%), thrombocytopenia (25%), and anemia (10%). The most frequent nonhematologic adverse events (any grade) included nausea (77%), infection (69%), fatigue (64%), diarrhea (42%), vomiting (40%), pyrexia (36%), constipation (31%), and anorexia (24%).
Conclusions: Single-agent bendamustine produced a high rate of objective responses with acceptable toxicity in patients with recurrent, rituximab-refractory indolent B-cell lymphoma.
Copyright 2010 American Cancer Society.
Conflict of interest statement
Conflict of Interest Disclosures: Research support was provided by Cephalon, Inc. Drs. Kahl, Bartlett, Leonard, Chen, Ganjoo, Williams, Czuczman, Robinson, Joyce, van der Hagt, and Cheson received research support from Cephalon, Inc. Dr. Chen is an employee of Cephalon, Inc. Dr. Leonard has acted as a consultant for Cephalon, Inc. Drs. Kahl, Leonard, Williams, Czuczman, and Cheson have served on advisory boards for Cephalon, Inc.
Figures
References
-
- Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. - PubMed
-
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. - PubMed
-
- Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–1992. - PubMed
-
- Foussard C, Mounier N, Van Hoof A, et al. Update of the FL2000 randomized trial combining rituximab to CHVP-interferon in follicular lymphoma patients [abstract] J Clin Oncol. 2006;18S(June 20 suppl):424. Abstract 7508.
-
- National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology. Non-Hodgkin's Lymphomas. Version 3.2008. Oct 0408. [January 13, 2009]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/nhl.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical