The Hofmeister effect on amyloid formation using yeast prion protein
- PMID: 19890987
- PMCID: PMC2817838
- DOI: 10.1002/pro.281
The Hofmeister effect on amyloid formation using yeast prion protein
Abstract
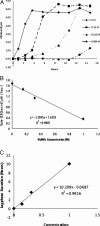
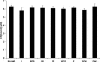
A variety of proteins are capable of converting from their soluble forms into highly ordered fibrous cross-beta aggregates (amyloids). This conversion is associated with certain pathological conditions in mammals, such as Alzheimer disease, and provides a basis for the infectious or hereditary protein isoforms (prions), causing neurodegenerative disorders in mammals and controlling heritable phenotypes in yeast. The N-proximal region of the yeast prion protein Sup35 (Sup35NM) is frequently used as a model system for amyloid conversion studies in vitro. Traditionally, amyloids are recognized by their ability to bind Congo Red dye specific to beta-sheet rich structures. However, methods for quantifying amyloid fibril formation thus far were based on measurements linking Congo Red absorbance to concentration of insulin fibrils and may not be directly applicable to other amyloid-forming proteins. Here, we present a corrected formula for measuring amyloid formation of Sup35NM by Congo Red assay. By utilizing this corrected procedure, we explore the effect of different sodium salts on the lag time and maximum rate of amyloid formation by Sup35NM. We find that increased kosmotropicity promotes amyloid polymerization in accordance with the Hofmeister series. In contrast, chaotropes inhibit polymerization, with the strength of inhibition correlating with the B-viscosity coefficient of the Jones-Dole equation, an increasingly accepted measure for the quantification of the Hofmeister series.
Figures
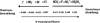



Similar articles
-
Ion-specific effects on prion nucleation and strain formation.J Biol Chem. 2013 Oct 18;288(42):30300-30308. doi: 10.1074/jbc.M113.467829. Epub 2013 Aug 29. J Biol Chem. 2013. PMID: 23990463 Free PMC article.
-
The physical dimensions of amyloid aggregates control their infective potential as prion particles.Elife. 2017 Sep 7;6:e27109. doi: 10.7554/eLife.27109. Elife. 2017. PMID: 28880146 Free PMC article.
-
Amyloid properties of the yeast cell wall protein Toh1 and its interaction with prion proteins Rnq1 and Sup35.Prion. 2019 Jan;13(1):21-32. doi: 10.1080/19336896.2018.1558763. Epub 2018 Dec 27. Prion. 2019. PMID: 30558459 Free PMC article.
-
Life cycle of yeast prions: propagation mediated by amyloid fibrils.Protein Pept Lett. 2009;16(3):271-6. doi: 10.2174/092986609787601796. Protein Pept Lett. 2009. PMID: 19275740 Review.
-
Probing the role of structural features of mouse PrP in yeast by expression as Sup35-PrP fusions.Prion. 2012 Jul 1;6(3):201-10. doi: 10.4161/pri.19214. Epub 2012 Jul 1. Prion. 2012. PMID: 22449853 Free PMC article. Review.
Cited by
-
Ionic liquids in protein amyloidogenesis: a brief screenshot of the state-of-the-art.Biophys Rev. 2018 Jun;10(3):847-852. doi: 10.1007/s12551-018-0425-4. Epub 2018 May 3. Biophys Rev. 2018. PMID: 29725930 Free PMC article.
-
Differences of cytotoxicity of orthodontic bands assessed by survival tests in Saccharomyces cerevisiae.Biomed Res Int. 2014;2014:143283. doi: 10.1155/2014/143283. Epub 2014 Jan 6. Biomed Res Int. 2014. PMID: 24511527 Free PMC article.
-
Anion Binding to Ammonium and Guanidinium Hosts: Implications for the Reverse Hofmeister Effects Induced by Lysine and Arginine Residues.J Org Chem. 2024 May 17;89(10):6877-6891. doi: 10.1021/acs.joc.4c00242. Epub 2024 Apr 25. J Org Chem. 2024. PMID: 38662908 Free PMC article.
-
Modulation of the Formation of Aβ- and Sup35NM-Based Amyloids by Complex Interplay of Specific and Nonspecific Ion Effects.J Phys Chem B. 2018 May 17;122(19):4972-4981. doi: 10.1021/acs.jpcb.7b12836. Epub 2018 May 3. J Phys Chem B. 2018. PMID: 29668283 Free PMC article.
-
Role of spatial ionic distribution on the energetics of hydrophobic assembly and properties of the water/hydrophobe interface.Phys Chem Chem Phys. 2012 Feb 14;14(6):1892-906. doi: 10.1039/c1cp20839j. Epub 2012 Jan 9. Phys Chem Chem Phys. 2012. PMID: 22231014 Free PMC article.
References
-
- Sipe JD, Cohen AS. Review: history of the amyloid fibril. J Struct Biol. 2000;130:88–98. - PubMed
-
- Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001;114:529–538. - PubMed
-
- Lai Z, Colon W, Kelly JW. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

