Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish
- PMID: 19891001
- PMCID: PMC3030279
- DOI: 10.1002/dvdy.22113
Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish
Abstract
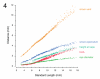
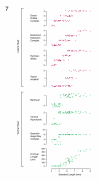
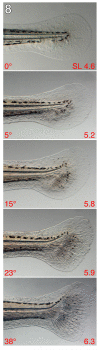
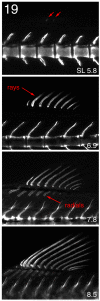
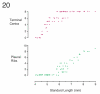
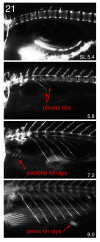
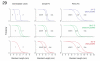
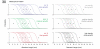
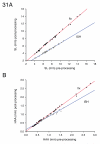
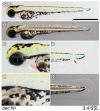
The zebrafish is a premier model organism yet lacks a system for assigning postembryonic fish to developmental stages. To provide such a staging series, we describe postembryonic changes in several traits that are visible under brightfield illumination or through vital staining and epiflourescent illumination. These include the swim bladder, median and pelvic fins, pigment pattern, scale formation, larval fin fold, and skeleton. We further identify milestones for placing postembryonic fish into discrete stages. We relate these milestones to changes in size and age and show that size is a better indicator of developmental progress than is age. We also examine how relationships between size and developmental progress vary with temperature and density, and we document the effects of histological processing on size. To facilitate postembryonic staging, we provide images of reference individuals that have attained specific developmental milestones and are of defined sizes. Finally, we provide guidelines for reporting stages that provide information on both discrete and continuous changes in growth and development.
(c) 2009 Wiley-Liss, Inc.
Figures


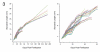


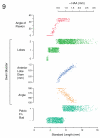


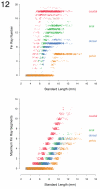


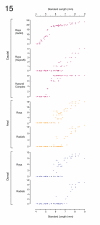


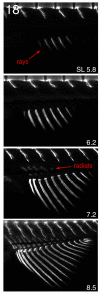



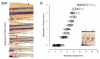




























References
-
- Balon EK. Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses) Environmental Biology Of Fishes. 1999;56:17–38.
-
- Bird NC, Mabee PM. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae) Dev Dyn. 2003;228:337–357. - PubMed
-
- Brown ME. Experimental studies on growth. In: Brown ME, editor. The Physiology of Fishes. Academic Press; New York: 1957. pp. 361–400.
-
- Campinho MA, Moutou KA, Power DM. Temperature sensitivity of skeletal ontogeny in Oreochromis mossambicus. Journal of Fish Biology. 2004;65:1003–1025.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

