A selective eradication of human nonhereditary breast cancer cells by phenanthridine-derived polyADP-ribose polymerase inhibitors
- PMID: 19891779
- PMCID: PMC2815540
- DOI: 10.1186/bcr2445
A selective eradication of human nonhereditary breast cancer cells by phenanthridine-derived polyADP-ribose polymerase inhibitors
Abstract
Introduction: PARP-1 (polyADP-ribose polymerase-1) is known to be activated in response to DNA damage, and activated PARP-1 promotes DNA repair. However, a recently disclosed alternative mechanism of PARP-1 activation by phosphorylated externally regulated kinase (ERK) implicates PARP-1 in a vast number of signal-transduction networks in the cell. Here, PARP-1 activation was examined for its possible effects on cell proliferation in both normal and malignant cells.
Methods: In vitro (cell cultures) and in vivo (xenotransplants) experiments were performed.
Results: Phenanthridine-derived PARP inhibitors interfered with cell proliferation by causing G2/M arrest in both normal (human epithelial cells MCF10A and mouse embryonic fibroblasts) and human breast cancer cells MCF-7 and MDA231. However, whereas the normal cells were only transiently arrested, G2/M arrest in the malignant breast cancer cells was permanent and was accompanied by a massive cell death. In accordance, treatment with a phenanthridine-derived PARP inhibitor prevented the development of MCF-7 and MDA231 xenotransplants in female nude mice. Quiescent cells (neurons and cardiomyocytes) are not impaired by these PARP inhibitors.
Conclusions: These results outline a new therapeutic approach for a selective eradication of abundant nonhereditary human breast cancers.
Figures
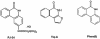




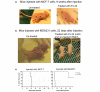
Comment in
-
PARP inhibitors and the treatment of breast cancer: beyond BRCA1/2?Breast Cancer Res. 2009;11(6):111. doi: 10.1186/bcr2451. Epub 2009 Nov 26. Breast Cancer Res. 2009. PMID: 20017885 Free PMC article.
References
-
- Cohen-Armon M. PARP-1 activation mediates the expression of immediate early genes implicated in long-term memory formation. FEBS J. 2008;275(Suppl 1):92.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

