Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart
- PMID: 19892241
- PMCID: PMC2800130
- DOI: 10.1016/j.jacc.2009.07.031
Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart
Abstract
Objectives: The aim of this study was to determine the impact of diabetes on oxidant balance and mitochondrial metabolism of carbohydrate- and lipid-based substrates in myocardium of type 2 diabetic patients.
Background: Heart failure represents a major cause of death among diabetic patients. It has been proposed that derangements in cardiac metabolism and oxidative stress may underlie the progression of this comorbidity, but scarce evidence exists in support of this mechanism in humans.
Methods: Mitochondrial oxygen (O(2)) consumption and hydrogen peroxide (H(2)O(2)) emission were measured in permeabilized myofibers prepared from samples of the right atrial appendage obtained from nondiabetic (n = 13) and diabetic (n = 11) patients undergoing nonemergent coronary artery bypass graft surgery.
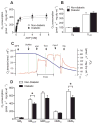
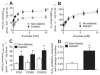
Results: Mitochondria in atrial tissue of type 2 diabetic individuals show a sharply decreased capacity for glutamate and fatty acid-supported respiration, in addition to an increased content of myocardial triglycerides, as compared to nondiabetic patients. Furthermore, diabetic patients show an increased mitochondrial H(2)O(2) emission during oxidation of carbohydrate- and lipid-based substrates, depleted glutathione, and evidence of persistent oxidative stress in their atrial tissue.
Conclusions: These findings are the first to directly investigate the effects of type 2 diabetes on a panoply of mitochondrial functions in the human myocardium using cellular and molecular approaches, and they show that mitochondria in diabetic human hearts have specific impairments in maximal capacity to oxidize fatty acids and glutamate, yet increased mitochondrial H(2)O(2) emission, providing insight into the role of mitochondrial dysfunction and oxidative stress in the pathogenesis of heart failure in diabetic patients.
2009 by the American College of Cardiology Foundation
Conflict of interest statement
Figures


Comment in
-
Alternative interpretation of mitochondrial metabolic changes in atrial tissue of type II diabetic human heart.J Am Coll Cardiol. 2010 Mar 23;55(12):1280-1281. doi: 10.1016/j.jacc.2009.12.022. J Am Coll Cardiol. 2010. PMID: 20298940 No abstract available.
References
-
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. - PubMed
-
- Saddik M, Lopaschuk GD. Triacylglycerol turnover in isolated working hearts of acutely diabetic rats. Can J Physiol Pharmacol. 1994;72:1110–9. - PubMed
-
- McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–5. - PubMed
-
- Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

