The rad52-Y66A allele alters the choice of donor template during spontaneous chromosomal recombination
- PMID: 19892607
- PMCID: PMC2818265
- DOI: 10.1016/j.dnarep.2009.10.001
The rad52-Y66A allele alters the choice of donor template during spontaneous chromosomal recombination
Abstract
Spontaneous mitotic recombination is a potential source of genetic changes such as loss of heterozygosity and chromosome translocations, which may lead to genetic disease. In this study we have used a rad52 hyper-recombination mutant, rad52-Y66A, to investigate the process of spontaneous heteroallelic recombination in the yeast Saccharomyces cerevisiae. We find that spontaneous recombination has different genetic requirements, depending on whether the recombination event occurs between chromosomes or between chromosome and plasmid sequences. The hyper-recombination phenotype of the rad52-Y66A mutation is epistatic with deletion of MRE11, which is required for establishment of DNA damage-induced cohesion. Moreover, single-cell analysis of strains expressing YFP-tagged Rad52-Y66A reveals a close to wild-type frequency of focus formation, but with foci lasting 6 times longer. This result suggests that spontaneous DNA lesions that require recombinational repair occur at the same frequency in wild-type and rad52-Y66A cells, but that the recombination process is slow in rad52-Y66A cells. Taken together, we propose that the slow recombinational DNA repair in the rad52-Y66A mutant leads to a by-pass of the window-of-opportunity for sister chromatid recombination normally promoted by MRE11-dependent damage-induced cohesion thereby causing a shift towards interchromosomal recombination.
Copyright (c) 2009 Elsevier B.V. All rights reserved.
Figures


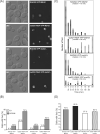
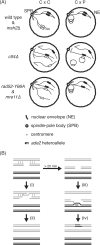
Similar articles
-
Control of Rad52 recombination activity by double-strand break-induced SUMO modification.Nat Cell Biol. 2006 Nov;8(11):1284-90. doi: 10.1038/ncb1488. Epub 2006 Oct 1. Nat Cell Biol. 2006. PMID: 17013376
-
Genome-wide analysis of Rad52 foci reveals diverse mechanisms impacting recombination.PLoS Genet. 2007 Dec;3(12):e228. doi: 10.1371/journal.pgen.0030228. PLoS Genet. 2007. PMID: 18085829 Free PMC article.
-
The ability of Sgs1 to interact with DNA topoisomerase III is essential for damage-induced recombination.DNA Repair (Amst). 2005 Feb 3;4(2):191-201. doi: 10.1016/j.dnarep.2004.09.002. DNA Repair (Amst). 2005. PMID: 15590327
-
Mechanics and Single-Molecule Interrogation of DNA Recombination.Annu Rev Biochem. 2016 Jun 2;85:193-226. doi: 10.1146/annurev-biochem-060614-034352. Epub 2016 Apr 18. Annu Rev Biochem. 2016. PMID: 27088880 Review.
-
Regulation of rDNA stability by sumoylation.DNA Repair (Amst). 2009 Apr 5;8(4):507-16. doi: 10.1016/j.dnarep.2009.01.015. Epub 2009 Mar 3. DNA Repair (Amst). 2009. PMID: 19261548 Review.
Cited by
-
RSC facilitates Rad59-dependent homologous recombination between sister chromatids by promoting cohesin loading at DNA double-strand breaks.Mol Cell Biol. 2011 Oct;31(19):3924-37. doi: 10.1128/MCB.01269-10. Epub 2011 Aug 1. Mol Cell Biol. 2011. PMID: 21807899 Free PMC article.
-
Histone H3K56 acetylation, Rad52, and non-DNA repair factors control double-strand break repair choice with the sister chromatid.PLoS Genet. 2013;9(1):e1003237. doi: 10.1371/journal.pgen.1003237. Epub 2013 Jan 24. PLoS Genet. 2013. PMID: 23357952 Free PMC article.
-
Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis.PLoS Biol. 2010 Oct 19;8(10):e1000520. doi: 10.1371/journal.pbio.1000520. PLoS Biol. 2010. PMID: 20976044 Free PMC article.
-
Rad52 sumoylation prevents the toxicity of unproductive Rad51 filaments independently of the anti-recombinase Srs2.PLoS Genet. 2013;9(10):e1003833. doi: 10.1371/journal.pgen.1003833. Epub 2013 Oct 10. PLoS Genet. 2013. PMID: 24130504 Free PMC article.
-
Enhancement of gene targeting in human cells by intranuclear permeation of the Saccharomyces cerevisiae Rad52 protein.Nucleic Acids Res. 2010 Aug;38(14):e149. doi: 10.1093/nar/gkq486. Epub 2010 Jun 2. Nucleic Acids Res. 2010. PMID: 20519199 Free PMC article.
References
-
- Game JC, Cox BS. Allelism tests of mutants affecting sensitivity to radiation in yeast and a proposed nomenclature. Mutat. Res. 1971;6:37–55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

