Binding of the human nucleotide excision repair proteins XPA and XPC/HR23B to the 5R-thymine glycol lesion and structure of the cis-(5R,6S) thymine glycol epimer in the 5'-GTgG-3' sequence: destabilization of two base pairs at the lesion site
- PMID: 19892827
- PMCID: PMC2811006
- DOI: 10.1093/nar/gkp844
Binding of the human nucleotide excision repair proteins XPA and XPC/HR23B to the 5R-thymine glycol lesion and structure of the cis-(5R,6S) thymine glycol epimer in the 5'-GTgG-3' sequence: destabilization of two base pairs at the lesion site
Abstract
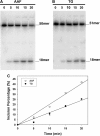
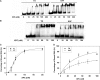
The 5R thymine glycol (5R-Tg) DNA lesion exists as a mixture of cis-(5R,6S) and trans-(5R,6R) epimers; these modulate base excision repair. We examine the 7:3 cis-(5R,6S):trans-(5R,6R) mixture of epimers paired opposite adenine in the 5'-GTgG-3' sequence with regard to nucleotide excision repair. Human XPA recognizes the lesion comparably to the C8-dG acetylaminoflourene (AAF) adduct, whereas XPC/HR23B recognition of Tg is superior. 5R-Tg is processed by the Escherichia coli UvrA and UvrABC proteins less efficiently than the C8-dG AAF adduct. For the cis-(5R, 6S) epimer Tg and A are inserted into the helix, remaining in the Watson-Crick alignment. The Tg N3H imine and A N(6) amine protons undergo increased solvent exchange. Stacking between Tg and the 3'-neighbor G*C base pair is disrupted. The solvent accessible surface and T(2) relaxation of Tg increases. Molecular dynamics calculations predict that the axial conformation of the Tg CH(3) group is favored; propeller twisting of the Tg*A pair and hydrogen bonding between Tg OH6 and the N7 atom of the 3'-neighbor guanine alleviate steric clash with the 5'-neighbor base pair. Tg also destabilizes the 5'-neighbor G*C base pair. This may facilitate flipping both base pairs from the helix, enabling XPC/HR23B recognition prior to recruitment of XPA.
Figures




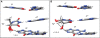
References
-
- Teoule R, Bonicel A, Bert C, Cadet J, Polverelli M. Identification of radioproducts resulting from the breakage of thymine moiety by gamma irradiation of E. coli DNA in an aerated aqueous solution. Radiat. Res. 1974;57:46–58. - PubMed
-
- Frenkel K, Goldstein MS, Teebor GW. Identification of the cis-thymine glycol moiety in chemically oxidized and gamma-irradiated deoxyribonucleic acid by high-pressure liquid chromatography analysis. Biochemistry. 1981;20:7566–7571. - PubMed
-
- Pfeifer GP. p53 mutational spectra and the role of methylated CpG sequences. Mutat. Res. 2000;450:155–166. - PubMed
-
- Vaishnav Y, Holwitt E, Swenberg C, Lee HC, Kan LS. Synthesis and characterization of stereoisomers of 5,6-dihydro-5,6-dihydroxy-thymidine. J. Biomol. Struct. Dyn. 1991;8:935–951. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

