Signal regulatory protein-beta1: a microglial modulator of phagocytosis in Alzheimer's disease
- PMID: 19893026
- PMCID: PMC2789620
- DOI: 10.2353/ajpath.2009.090147
Signal regulatory protein-beta1: a microglial modulator of phagocytosis in Alzheimer's disease
Abstract
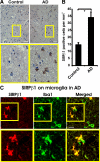
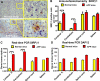
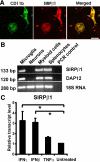
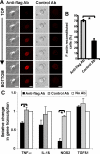
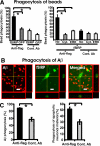
The signal regulatory protein-beta1 (SIRPbeta1) is a DAP12-associated transmembrane receptor expressed in a subset of hematopoietic cells. Recently, it was shown that peritoneal macrophages express SIRPbeta1, which positively regulated phagocytosis. Here, we found that SIRPbeta1 was up-regulated and acted as a phagocytic receptor on microglia in amyloid precursor protein J20 (APP/J20) transgenic mice and in Alzheimer's disease (AD) patients. Interferon (IFN)-gamma and IFN-beta stimulated gene transcription of SIRPbeta1 in cultured microglia. Activation of SIRPbeta1 on cultured microglia by cross-linking antibodies induced reorganization of the cytoskeleton protein beta-actin and suppressed lipopolysaccharide-induced gene transcription of tumor necrosis factor-alpha and nitric oxide synthase-2. Furthermore, activation of SIRPbeta1 increased phagocytosis of microsphere beads, neural debris, and fibrillary amyloid-beta (Abeta). Phagocytosis of neural cell debris and Abeta was impaired after lentiviral knockdown of SIRPbeta1 in primary microglial cells. Thus, SIRPbeta1 is a novel IFN-induced microglial receptor that supports clearance of neural debris and Abeta aggregates by stimulating phagocytosis.
Figures


References
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. - PubMed
-
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal “on” and “off” signals control microglia. Trends Neurosci. 2007;30:596–602. - PubMed
-
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. - PubMed
-
- Streit JM. Kettenmann H, Ransome B R, editors. Oxford, UK: Oxford University Press,; Microglial cells in Neuroglia second edition, 2005:pp 60–71.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

