Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis
- PMID: 19893030
- PMCID: PMC2789600
- DOI: 10.2353/ajpath.2009.090166
Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis
Abstract
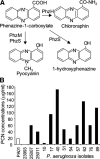
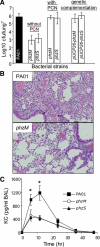
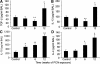
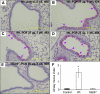
The cystic fibrosis (CF) airway bacterial pathogen Pseudomonas aeruginosa secretes multiple virulence factors. Among these, the redox active exotoxin pyocyanin (PCN) is produced in concentrations up to 100 mumol/L during infection of CF and other bronchiectatic airways. However, the contributions of PCN during infection of bronchiectatic airways are not appreciated. In this study, we demonstrate that PCN is critical for chronic infection in mouse airways and orchestrates adaptive immune responses that mediate lung damage. Wild-type FVBN mice chronically exposed to PCN developed goblet cell hyperplasia and metaplasia, airway fibrosis, and alveolar airspace destruction. Furthermore, after 12 weeks of exposure to PCN, mouse lungs down-regulated the expression of T helper (Th) type 1 cytokines and polarized toward a Th2 response. Cellular analyses indicated that chronic exposure to PCN profoundly increased the lung population of recruited macrophages, CD4(+) T cells, and neutrophils responsible for the secretion of these cytokines. PCN-mediated goblet cell hyperplasia and metaplasia required Th2 cytokine signaling through the Stat6 pathway. In summary, this study establishes that PCN is an important P. aeruginosa virulence factor capable of directly inducing pulmonary pathophysiology in mice, consistent with changes observed in CF and other bronchiectasis lungs.
Figures



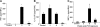
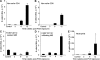

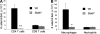
References
-
- Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. - PubMed
-
- Lau GW, Ran H, Hassett DJ, Kong FS. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606. - PubMed
-
- Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 2005;13:389–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

