Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice
- PMID: 19893049
- PMCID: PMC2789601
- DOI: 10.2353/ajpath.2009.081096
Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice
Abstract
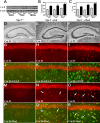
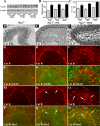
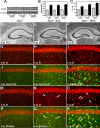
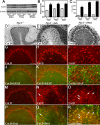
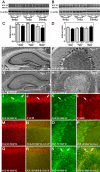
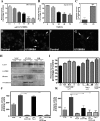
Niemann-Pick disease type C (NPC), caused by mutations in the Npc1 or Npc2 genes, is a progressive neurodegenerative disorder characterized by intracellular accumulation/redistribution of cholesterol in a number of tissues including the brain. This is accompanied by a severe loss of neurons in selected brain regions. In this study, we evaluated the role of lysosomal enzymes, cathepsins B and D, in determining neuronal vulnerability in NPC1-deficient (Npc1(-/-)) mouse brains. Our results showed that Npc1(-/-) mice exhibit an age-dependent degeneration of neurons in the cerebellum but not in the hippocampus. The cellular level/expression and activity of cathepsins B and D are increased more predominantly in the cerebellum than in the hippocampus of Npc1(-/-) mice. In addition, the cytosolic levels of cathepsins, cytochrome c, and Bax2 are higher in the cerebellum than in the hippocampus of Npc1(-/-) mice, suggesting a role for these enzymes in the degeneration of neurons. This suggestion is supported by our observation that degeneration of cultured cortical neurons treated with U18666A, which induces an NPC1-like phenotype at the cellular level, can be attenuated by inhibition of cathepsin B or D enzyme activity. These results suggest that the increased level/activity and altered subcellular distribution of cathepsins may be associated with the underlying cause of neuronal vulnerability in Npc1(-/-) brains. Therefore, their inhibitors may have therapeutic potential in attenuating NPC pathology.
Figures


References
-
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson M, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. - PubMed
-
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. - PubMed
-
- Vanier MT, Millat G. Niemann-Pick disease type C. Clin Genet. 2003;64:269–281. - PubMed
-
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. - PubMed
-
- Pentchev PV, Vanier MT, Suzuki K, Patterson MC. Niemann-Pick disease type C: a cellular cholesterol lipidosis. Scriver CR, Beaudet AL, Sly WD, Valle D, editors. New York: McGraw-Hill,; 1995:pp 2625–2639.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

