Tipin/Tim1/And1 protein complex promotes Pol alpha chromatin binding and sister chromatid cohesion
- PMID: 19893489
- PMCID: PMC2775894
- DOI: 10.1038/emboj.2009.304
Tipin/Tim1/And1 protein complex promotes Pol alpha chromatin binding and sister chromatid cohesion
Abstract
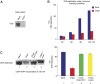
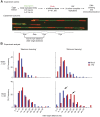
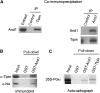
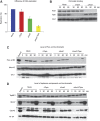
The Tipin/Tim1 complex plays an important role in the S-phase checkpoint and replication fork stability. However, the biochemical function of this complex is poorly understood. Using Xenopus laevis egg extract we show that Tipin is required for DNA replication in the presence of limiting amount of replication origins. Under these conditions the DNA replication defect correlates with decreased levels of DNA Polalpha on chromatin. We identified And1, a Polalpha chromatin-loading factor, as new Tipin-binding partner. We found that both Tipin and And1 promote stable binding of Polalpha to chromatin and that this is required for DNA replication under unchallenged conditions. Strikingly, extracts lacking Tipin and And1 also show reduced sister chromatids cohesion. These data indicate that Tipin/Tim1/And1 form a complex that links stabilization of replication fork and establishment of sister chromatid cohesion.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Ben-Shahar TR, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F (2008) Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321: 563–566 - PubMed
-
- Branzei D, Foiani M (2005) The DNA damage response during DNA replication. Curr Opin Cell Biol 17: 568–575 - PubMed
-
- Chan RC, Chan A, Jeon M, Wu TF, Pasqualone D, Rougvie AE, Meyer BJ (2003) Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature 423: 1002–1009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

