The germ cell nuclear proteins hnRNP G-T and RBMY activate a testis-specific exon
- PMID: 19893608
- PMCID: PMC2762042
- DOI: 10.1371/journal.pgen.1000707
The germ cell nuclear proteins hnRNP G-T and RBMY activate a testis-specific exon
Abstract
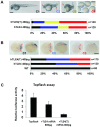
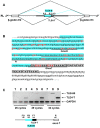
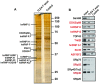
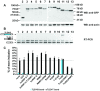
The human testis has almost as high a frequency of alternative splicing events as brain. While not as extensively studied as brain, a few candidate testis-specific splicing regulator proteins have been identified, including the nuclear RNA binding proteins RBMY and hnRNP G-T, which are germ cell-specific versions of the somatically expressed hnRNP G protein and are highly conserved in mammals. The splicing activator protein Tra2beta is also highly expressed in the testis and physically interacts with these hnRNP G family proteins. In this study, we identified a novel testis-specific cassette exon TLE4-T within intron 6 of the human transducing-like enhancer of split 4 (TLE4) gene which makes a more transcriptionally repressive TLE4 protein isoform. TLE4-T splicing is normally repressed in somatic cells because of a weak 5' splice site and surrounding splicing-repressive intronic regions. TLE4-T RNA pulls down Tra2beta and hnRNP G proteins which activate its inclusion. The germ cell-specific RBMY and hnRNP G-T proteins were more efficient in stimulating TLE4-T incorporation than somatically expressed hnRNP G protein. Tra2b bound moderately to TLE4-T RNA, but more strongly to upstream sites to potently activate an alternative 3' splice site normally weakly selected in the testis. Co-expression of Tra2beta with either hnRNP G-T or RBMY re-established the normal testis physiological splicing pattern of this exon. Although they can directly bind pre-mRNA sequences around the TLE4-T exon, RBMY and hnRNP G-T function as efficient germ cell-specific splicing co-activators of TLE4-T. Our study indicates a delicate balance between the activity of positive and negative splicing regulators combinatorially controls physiological splicing inclusion of exon TLE4-T and leads to modulation of signalling pathways in the testis. In addition, we identified a high-affinity binding site for hnRNP G-T protein, showing it is also a sequence-specific RNA binding protein.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
RBMY, a probable human spermatogenesis factor, and other hnRNP G proteins interact with Tra2beta and affect splicing.Hum Mol Genet. 2000 Mar 22;9(5):685-94. doi: 10.1093/hmg/9.5.685. Hum Mol Genet. 2000. PMID: 10749975
-
Binding of hnRNP H and U2AF65 to respective G-codes and a poly-uridine tract collaborate in the N50-5'ss selection of the REST N exon in H69 cells.PLoS One. 2012;7(7):e40315. doi: 10.1371/journal.pone.0040315. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792276 Free PMC article.
-
The RNA binding protein hnRNP Q modulates the utilization of exon 7 in the survival motor neuron 2 (SMN2) gene.Mol Cell Biol. 2008 Nov;28(22):6929-38. doi: 10.1128/MCB.01332-08. Epub 2008 Sep 15. Mol Cell Biol. 2008. PMID: 18794368 Free PMC article.
-
Evolution of SR protein and hnRNP splicing regulatory factors.Wiley Interdiscip Rev RNA. 2012 Jan-Feb;3(1):1-12. doi: 10.1002/wrna.100. Epub 2011 Sep 2. Wiley Interdiscip Rev RNA. 2012. PMID: 21898828 Free PMC article. Review.
-
How does Tra2β protein regulate tissue-specific RNA splicing?Biochem Soc Trans. 2012 Aug;40(4):784-8. doi: 10.1042/BST20120036. Biochem Soc Trans. 2012. PMID: 22817734 Free PMC article. Review.
Cited by
-
Transcriptional factors smad1 and smad9 act redundantly to mediate zebrafish ventral specification downstream of smad5.J Biol Chem. 2014 Mar 7;289(10):6604-6618. doi: 10.1074/jbc.M114.549758. Epub 2014 Jan 31. J Biol Chem. 2014. PMID: 24488494 Free PMC article.
-
A candidate protective factor in amyotrophic lateral sclerosis: heterogenous nuclear ribonucleoprotein G.Neural Regen Res. 2023 Jul;18(7):1527-1534. doi: 10.4103/1673-5374.357916. Neural Regen Res. 2023. PMID: 36571358 Free PMC article.
-
Differential RNA-binding activity of the hnRNP G protein correlated with the sex genotype in the amphibian oocyte.Nucleic Acids Res. 2011 May;39(10):4109-21. doi: 10.1093/nar/gkq1315. Epub 2011 Jan 29. Nucleic Acids Res. 2011. PMID: 21278421 Free PMC article.
-
The RNA recognition motif protein RBM11 is a novel tissue-specific splicing regulator.Nucleic Acids Res. 2012 Feb;40(3):1021-32. doi: 10.1093/nar/gkr819. Epub 2011 Oct 7. Nucleic Acids Res. 2012. PMID: 21984414 Free PMC article.
-
An ancient germ cell-specific RNA-binding protein protects the germline from cryptic splice site poisoning.Elife. 2019 Jan 24;8:e39304. doi: 10.7554/eLife.39304. Elife. 2019. PMID: 30674417 Free PMC article.
References
-
- Wang HY, Xu X, Ding JH, Bermingham JR, , Jr., Fu XD. SC35 plays a role in T cell development and alternative splicing of CD45. Mol Cell. 2001;7:331–342. - PubMed
-
- Xu XD, Yang DM, Ding JH, Wang W, Chu PH, et al. ASF/SF2-Regulated CaMKII delta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120:59–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

